Mutational analysis of Candida albicans SNF7 reveals genetically separable Rim101 and ESCRT functions and demonstrates divergence in bro1-domain protein interactions
- PMID: 20026677
- PMCID: PMC2845337
- DOI: 10.1534/genetics.109.112029
Mutational analysis of Candida albicans SNF7 reveals genetically separable Rim101 and ESCRT functions and demonstrates divergence in bro1-domain protein interactions
Abstract
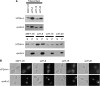
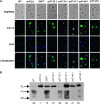
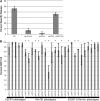
The opportunistic pathogen Candida albicans can grow over a wide pH range, which is associated with its ability to colonize and infect distinct host niches. C. albicans growth in neutral-alkaline environments requires proteolytic activation of the transcription factor Rim101. Rim101 activation requires Snf7, a member of the endosomal sorting complex required for transport (ESCRT) pathway. We hypothesized that Snf7 has distinct functions in the Rim101 and ESCRT pathways, which we tested by alanine-scanning mutagenesis. While some snf7 alleles conferred no defects, we identified alleles with solely ESCRT-dependent, solely Rim101-dependent, or both Rim101- and ESCRT-dependent defects. Thus, Snf7 function in these two pathways is at least partially separable. Both Rim101- and ESCRT-dependent functions require Snf7 recruitment to the endosomal membrane and alleles that disrupted both pathways were found to localize normally, suggesting a downstream defect. Most alleles that conferred solely Rim101-dependent defects were still able to process Rim101 normally under steady-state conditions. However, these same strains did display a kinetic defect in Rim101 processing. Several alleles with solely Rim101-dependent defects mapped to the C-terminal end of Snf7. Further analyses suggested that these mutations disrupted interactions with bro-domain proteins, Rim20 and Bro1, in overlapping but slightly divergent Snf7 domains.
Figures



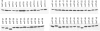


References
-
- Adams, A., D. E. Gottschling, C. A. Kaiser and T. Stearns (Editors), 1997. Methods in Yeast Genetics, 1997: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo and S. D. Emr, 2002. a Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3 271–282. - PubMed
-
- Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland and S. D. Emr, 2002. b Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3 283–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

