Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals
- PMID: 20026682
- PMCID: PMC2857868
- DOI: 10.1096/fj.09-149153
Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals
Abstract
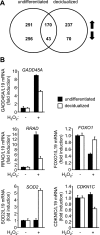
Survival of the conceptus is dependent on continuous progesterone signaling in the maternal decidua but how this is achieved under conditions of oxidative stress that characterize early pregnancy is unknown. Using primary cultures, we show that modest levels of reactive oxygen species (ROS) increase sumoylation in human endometrial stromal cells (HESCs), leading to enhanced modification and transcriptional inhibition of the progesterone receptor (PR). The ability of ROS to induce a sustained hypersumoylation response, or interfere with PR activity, was lost upon differentiation of HESCs into decidual cells. Hypersumoylation in response to modest levels of ROS requires activation of the JNK pathway. Although ROS-dependent JNK signaling is disabled on decidualization, the cells continue to mount a transcriptional response, albeit distinct from that observed in undifferentiated HESCs. We further show that attenuated JNK signaling in decidual cells is a direct consequence of altered expression of key pathway modulators, including induction of MAP kinase phosphatase 1 (MKP1). Overexpression of MKP1 dampens JNK signaling, prevents hypersumoylation, and maintains PR activity in undifferentiated HESCs exposed to ROS. Thus, JNK silencing uncouples ROS signaling from the SUMO conjugation pathway and maintains progesterone responses and cellular homeostasis in decidual cells under oxidative stress conditions imposed by pregnancy.
Figures

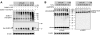


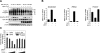
References
-
- Brosens J J, Gellersen B. Death or survival–progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. - PubMed
-
- Labied S, Kajihara T, Madureira P A, Fusi L, Jones M C, Higham J M, Varshochi R, Francis J M, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam E W, Brosens J J. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. - PubMed
-
- Critchley H O, Kelly R W, Brenner R M, Baird D T. The endocrinology of menstruation—a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. - PubMed
-
- Gellersen B, Brosens I A, Brosens J J. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. - PubMed
-
- Brosens J J, Parker M G, McIndoe A, Pijnenborg R, Brosens I A. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200:615.e1–615.e6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

