Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure
- PMID: 20026781
- PMCID: PMC2810546
- DOI: 10.1161/CIRCULATIONAHA.109.869396
Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure
Abstract
Background: Patients after the Fontan procedure are at risk for suboptimal functional health status, and associations with laboratory measures are important for planning interventions and outcome measures for clinical trials.
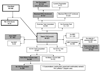
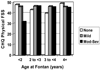
Methods and results: Parents completed the generic Child Health Questionnaire for 511 Fontan Cross-Sectional Study patients 6 to 18 years of age (61% male). Associations of Child Health Questionnaire Physical and Psychosocial Functioning Summary Scores (FSS) with standardized measurements from prospective exercise testing, echocardiography, magnetic resonance imaging, and measurement of brain natriuretic peptide were determined by regression analyses. For exercise variables for maximal effort patients only, the final model showed that higher Physical FSS was associated only with higher maximum work rate, accounting for 9% of variation in Physical FSS. For echocardiography, lower Tei index (particularly for patients with extracardiac lateral tunnel connections), lower indexed end-systolic volume, and the absence of atrioventricular valve regurgitation for patients having Fontan procedure at age <2 years were associated with higher Physical FSS, accounting for 14% of variation in Physical FSS. For magnetic resonance imaging, ratio of lower mass to end-diastolic volume and midquartiles of indexed end-systolic volume (nonlinear) were associated with higher Physical FSS, accounting for 11% of variation. Lower brain natriuretic peptide was significantly but weakly associated with higher Physical FSS (1% of variation). Significant associations for Psychosocial FSS with laboratory measures were fewer and weaker than for Physical FSS.
Conclusions: In relatively healthy Fontan patients, laboratory measures account for a small proportion of the variation in functional health status and therefore may not be optimal surrogate end points for trials of therapeutic interventions.
Figures

Similar articles
-
Functional state of patients with heterotaxy syndrome following the Fontan operation.Cardiol Young. 2007 Sep;17 Suppl 2:44-53. doi: 10.1017/S1047951107001151. Cardiol Young. 2007. PMID: 18039398
-
Factors associated with serum brain natriuretic peptide levels after the Fontan procedure.Congenit Heart Dis. 2011 Jul-Aug;6(4):313-21. doi: 10.1111/j.1747-0803.2011.00496.x. Epub 2011 Mar 25. Congenit Heart Dis. 2011. PMID: 21435188 Free PMC article.
-
Relation of size of secondary ventricles to exercise performance in children after fontan operation.Am J Cardiol. 2010 Dec 1;106(11):1652-6. doi: 10.1016/j.amjcard.2010.07.026. Am J Cardiol. 2010. PMID: 21094369 Free PMC article.
-
3-Month Enalapril Treatment in Pediatric Fontan Patients With Moderate to Good Systolic Ventricular Function.Am J Cardiol. 2022 Jan 15;163:98-103. doi: 10.1016/j.amjcard.2021.10.013. Epub 2021 Nov 10. Am J Cardiol. 2022. PMID: 34774285
-
Exercise testing after the Fontan operation.Pediatr Cardiol. 1999 Jan-Feb;20(1):57-9; discussion 60. doi: 10.1007/s002469900397. Pediatr Cardiol. 1999. PMID: 9861079 Review. No abstract available.
Cited by
-
Exercise capacity after total cavopulmonary anastomosis: a longitudinal paediatric and adult study.ESC Heart Fail. 2022 Feb;9(1):337-344. doi: 10.1002/ehf2.13747. Epub 2021 Dec 10. ESC Heart Fail. 2022. PMID: 34894102 Free PMC article.
-
Association of Habitual Activity and Body Mass Index in Survivors of Congenital Heart Surgery: A Study of Children and Adolescents With Tetralogy of Fallot, Transposition of the Great Arteries, and Fontan Palliation.World J Pediatr Congenit Heart Surg. 2018 Mar;9(2):177-184. doi: 10.1177/2150135117752122. World J Pediatr Congenit Heart Surg. 2018. PMID: 29544424 Free PMC article.
-
Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation.Circ Cardiovasc Imaging. 2014 May;7(3):502-9. doi: 10.1161/CIRCIMAGING.113.001473. Epub 2014 Mar 11. Circ Cardiovasc Imaging. 2014. PMID: 24619103 Free PMC article.
-
Assessment of Quality of Life in Young Patients with Single Ventricle after the Fontan Operation.J Pediatr. 2016 Mar;170:166-72.e1. doi: 10.1016/j.jpeds.2015.11.016. Epub 2015 Dec 10. J Pediatr. 2016. PMID: 26685073 Free PMC article.
-
Pediatric Exercise Testing: Value and Implications of Peak Oxygen Uptake.Children (Basel). 2017 Jan 24;4(1):6. doi: 10.3390/children4010006. Children (Basel). 2017. PMID: 28125022 Free PMC article. Review.
References
-
- Freedom RM, Hamilton R, Yoo SJ, Mikailian H, Benson L, McCrindle B, Justino H, Williams WG. The Fontan procedure: analysis of cohorts and late complications. Cardiol Young. 2000;10:307–331. - PubMed
-
- Giardini A, Napoleone CP, Specchia S, Donti A, Formigari R, Oppido G, Gargiulo G, Picchio FM. Conversion of atriopulmonary Fontan to extracardiac total cavopulmonary connection improves cardiopulmonary function. Int J Cardiol. 2006;113:341–344. - PubMed
-
- Azakie A, McCrindle BW, Van Arsdell G, Benson LN, Coles J, Hamilton R, Freedom RM, Williams WG. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg. 2001;122:1219–1228. - PubMed
-
- Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after fontan procedures--the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. 1998;115:493–498. - PubMed
-
- Kouatli AA, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation. 1997;96:1507–1512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL068288/HL/NHLBI NIH HHS/United States
- HL068285/HL/NHLBI NIH HHS/United States
- U01 HL068269/HL/NHLBI NIH HHS/United States
- U01 HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068290/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- U10 HL109778/HL/NHLBI NIH HHS/United States
- HL068290/HL/NHLBI NIH HHS/United States
- HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068288/HL/NHLBI NIH HHS/United States
- HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068292/HL/NHLBI NIH HHS/United States
- HL068269/HL/NHLBI NIH HHS/United States
- HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068285/HL/NHLBI NIH HHS/United States
- HL068292/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

