Molecular insights into mu opioid pharmacology: From the clinic to the bench
- PMID: 20026962
- PMCID: PMC2810866
- DOI: 10.1097/AJP.0b013e3181c49d2e
Molecular insights into mu opioid pharmacology: From the clinic to the bench
Abstract
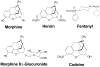
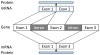
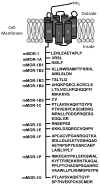
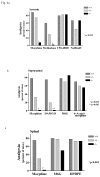
Most of the opioids used in clinical practice exert their effects through mu opioid receptors. Yet, subtle but important pharmacological differences have been observed among the mu opioids. Their potency, effectiveness, and adverse effects can vary unpredictably among patients. These clinical differences among the mu opioids strongly argue against a single receptor mediating their actions. The cloning of the mu opioid receptor has greatly enhanced our understanding of the complexity of this system and has provided possible mechanisms to explain these observations. A single mu opioid receptor gene has been identified, but we now know that it generates a multitude of different mu opioid receptor subtypes through a mechanism commonly used to enhance protein diversity, alternative splicing. Early studies identified a number of splice variants involving the tip of the C-terminus. This region of the receptor is far away from the binding pocket, explaining why these variants still exhibit the same selectivity for mu opioids. However, the differences in structure at the C-terminus influence the activation patterns of the mu opioids. In addition, a second series of variants has been isolated that involves alternative splicing at the N-terminus. Together, these sets of mu opioid receptor splice variants may help explain the clinical variability of the mu drugs among patients and provide insights into why it is so important to individualize therapy for every patient in pain.
Conflict of interest statement
Disclosure: Dr. Pasternak reports no conflicts of interest related to this paper.
Figures

References
-
- Mayer DJ, Liebeskind JC. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 1974;68(1):73–93. - PubMed
-
- Lord JA, Waterfield AA, Hughes J, et al. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267(5611):495–499. - PubMed
-
- Martin WR, Eades CG, Thompson JA, et al. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197(3):517–532. - PubMed
-
- Pasternak GW, Bodnar RJ, Clark JA, et al. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41(26):2845–2849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

