Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling
- PMID: 20027123
- PMCID: PMC2929929
- DOI: 10.1097/HJH.0b013e3283358b6e
Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling
Abstract
Objective: The mechanisms by which inflammation activates sympathetic drive in heart failure and hypertension remain ill-defined. In this study, an intracerebroventricular injection of lipopolysaccharide (LPS) was used to induce the expression of cytokines and other inflammatory mediators in the brain, in the absence of other excitatory mediators, and the downstream signaling pathways leading to sympathetic activation were examined using intracerebroventricular injections of blocking or inhibiting agents.
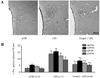
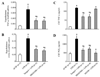
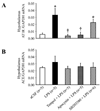
Methods and results: In anesthetized rats, intracerebroventricular injection of LPS (5 microg) increased (P < 0.05) renal sympathetic nerve activity, blood pressure and heart rate. LPS increased (P < 0.05) hypothalamic mRNA for NAD(P)H oxidase subunits p47 and gp91, NAD(P)H oxidase-dependent superoxide generation, hypothalamic mRNA for tumor necrosis factor-alpha, cyclooxygenase-2 and cerebrospinal fluid levels of tumor necrosis factor-alpha and prostaglandin E2. In the paraventricular nucleus of hypothalamus, dihydroethidium staining for superoxide expression and c-Fos activity (indicating neuronal excitation) increased. The superoxide scavenger tempol significantly (P < 0.05) diminished the expression of inflammatory mediators, as well as superoxide expression and neuronal excitation in paraventricular nucleus. SB203580 (p38 mitogen-activated protein kinase inhibitor) also reduced the expression of inflammatory mediators in hypothalamus and cerebrospinal fluid. Tempol, apocynin [NAD(P)H oxidase inhibitor], SB203580 and NS398 (cyclooxygenase-2 inhibitor) all reduced cerebrospinal fluid prostaglandin E2 and the sympathoexcitatory response to LPS. LPS also increased angiotensin II type 1 receptor mRNA, a response blocked by apocynin and tempol but not by SB203580.
Conclusion: These findings suggest that central inflammation in pathophysiological conditions activates the sympathetic nervous system via NAD(P)H oxidase and p38 mitogen-activated protein kinase-dependent synthesis of prostaglandin E2.
Keywords: inflammation; lipopolysaccharide; oxidative stress; paraventricular nucleus of hypothalamus; prostaglandins.
Figures




Similar articles
-
Superoxide anions modulate the performance of apelin in the paraventricular nucleus on sympathetic activity and blood pressure in spontaneously hypertensive rats.Peptides. 2019 Nov;121:170051. doi: 10.1016/j.peptides.2018.12.005. Epub 2018 Dec 21. Peptides. 2019. PMID: 30582943
-
Angiotensin-(1-7) in Paraventricular Nucleus Contributes to the Enhanced Cardiac Sympathetic Afferent Reflex and Sympathetic Activity in Chronic Heart Failure Rats.Cell Physiol Biochem. 2017;42(6):2523-2539. doi: 10.1159/000480214. Epub 2017 Aug 23. Cell Physiol Biochem. 2017. PMID: 28848201 Free PMC article.
-
Acute pressor effect of central angiotensin II is mediated by NAD(P)H-oxidase-dependent production of superoxide in the hypothalamic cardiovascular regulatory nuclei.J Hypertens. 2006 Jan;24(1):109-16. doi: 10.1097/01.hjh.0000198026.99600.59. J Hypertens. 2006. PMID: 16331108
-
Vagal afferents, sympathetic efferents and the role of the PVN in heart failure.Auton Neurosci. 2016 Aug;199:38-47. doi: 10.1016/j.autneu.2016.08.009. Epub 2016 Aug 9. Auton Neurosci. 2016. PMID: 27522531 Review.
-
Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure.Exp Physiol. 2010 Jan;95(1):19-25. doi: 10.1113/expphysiol.2008.045948. Epub 2009 Jul 31. Exp Physiol. 2010. PMID: 19648480 Free PMC article. Review.
Cited by
-
Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus.Am J Physiol Regul Integr Comp Physiol. 2014 Mar 1;306(5):R328-40. doi: 10.1152/ajpregu.00506.2013. Epub 2013 Dec 31. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24381176 Free PMC article.
-
Blocking p38 Signaling Reduces the Activation of Pro-inflammatory Cytokines and the Phosphorylation of p38 in the Habenula and Reverses Depressive-Like Behaviors Induced by Neuroinflammation.Front Pharmacol. 2018 May 15;9:511. doi: 10.3389/fphar.2018.00511. eCollection 2018. Front Pharmacol. 2018. PMID: 29867510 Free PMC article.
-
Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension.Biomed Res Int. 2014;2014:406960. doi: 10.1155/2014/406960. Epub 2014 Jul 20. Biomed Res Int. 2014. PMID: 25136585 Free PMC article. Review.
-
Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis.Steroids. 2014 Dec;91:20-31. doi: 10.1016/j.steroids.2014.08.014. Steroids. 2014. PMID: 25173821 Free PMC article. Review.
-
Angiotensin II Receptor Blockers Attenuate Lipopolysaccharide-Induced Memory Impairment by Modulation of NF-κB-Mediated BDNF/CREB Expression and Apoptosis in Spontaneously Hypertensive Rats.Mol Neurobiol. 2018 Feb;55(2):1725-1739. doi: 10.1007/s12035-017-0450-5. Epub 2017 Feb 18. Mol Neurobiol. 2018. PMID: 28215000
References
-
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. - PubMed
-
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. - PubMed
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. - PubMed
-
- Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

