Mechanisms guiding primordial germ cell migration: strategies from different organisms
- PMID: 20027186
- PMCID: PMC4521894
- DOI: 10.1038/nrm2815
Mechanisms guiding primordial germ cell migration: strategies from different organisms
Abstract
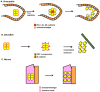
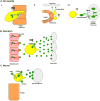
The regulated migration of cells is essential for development and tissue homeostasis, and aberrant cell migration can lead to an impaired immune response and the progression of cancer. Primordial germ cells (PGCs), precursors to sperm and eggs, have to migrate across the embryo to reach somatic gonadal precursors, where they carry out their function. Studies of model organisms have revealed that, despite important differences, several features of PGC migration are conserved. PGCs require an intrinsic motility programme and external guidance cues to survive and successfully migrate. Proper guidance involves both attractive and repulsive cues and is mediated by protein and lipid signalling.
Figures

References
-
- Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. - PubMed
-
- Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–6. - PubMed
-
- Webb DJ, Zhang H, Horwitz AF. Cell migration: an overview. Methods Mol Biol. 2005;294:3–11. - PubMed
-
- Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell. 2002;2:153–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

