Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra
- PMID: 20027219
- PMCID: PMC2791211
- DOI: 10.1371/journal.pone.0008268
Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra
Abstract
Background: Vitamin B12 is indispensable for proper brain functioning and cytosolic synthesis of S-adenosylmethionine. Whether its deficiency produces effects on viability and apoptosis of neurons remains unknown. There is a particular interest in investigating these effects in Parkinson disease where Levodopa treatment is known to increase the consumption of S-adenosylmethionine. To cause deprivation of vitamin B12, we have recently developed a cell model that produces decreased synthesis of S-adenosylmethionine by anchoring transcobalamin (TCII) to the reticulum through its fusion with Oleosin (OLEO).
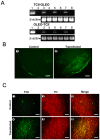
Methodology: Gene constructs including transcobalamin-oleosin (TCII-OLEO) and control constructs, green fluorescent protein-transcobalamin-oleosin (GFP-TCII-OLEO), oleosin-transcobalamin (OLEO-TCII), TCII and OLEO were used for expression in N1E-115 cells (mouse neuroblastoma) and in substantia nigra of adult rats, using a targeted transfection with a Neurotensin polyplex system. We studied the viability and the apoptosis in the transfected cells and targeted tissue. The turning behavior was evaluated in the rats transfected with the different plasmids.
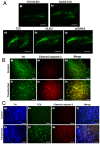
Principal findings: The transfection of N1E-115 cells by the TCII-OLEO-expressing plasmid significantly affected cell viability and increased immunoreactivity of cleaved Caspase-3. No change in propidium iodide uptake (used as a necrosis marker) was observed. The transfected rats lost neurons immunoreactive to tyrosine hydroxylase. The expression of TCII-OLEO was observed in cells immunoreactive to tyrosine hydroxylase of the substantia nigra, with a superimposed expression of cleaved Caspase-3. These cellular and tissular effects were not observed with the control plasmids. Rats transfected with TCII-OLEO expressing plasmid presented with a significantly higher number of turns, compared with those transfected with the other plasmids.
Conclusions/significance: In conclusion, the TCII-OLEO transfection was responsible for apoptosis in N1E-115 cells and rat substantia nigra and for Parkinson-like phenotype. This suggests evaluating whether vitamin B12 deficit could aggravate the PD in patients under Levodopa therapy by impairing S-adenosylmethionine synthesis in substantia nigra.
Conflict of interest statement
Figures




References
-
- Russell JSR, Batten FE, Collier J. Subacute combined degeneration of the spinal cord. Brain. 1900;23:39–110.
-
- Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog Neurobiol. 2009;88:203–20. - PubMed
-
- Forges T, Monnier-Barbarino P, Alberto JM, Guéant-Rodriguez RM, Daval JL, et al. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–38. - PubMed
-
- Cheng H, Gomes-Trolin C, Aquilonius SM, Steinberg A, Löfberg C, et al. Levels of L-methionine S-adenosyltransferase activity in erythrocytes and concentrations of S-adenosylmethionine and S-adenosylhomocysteine in whole blood of patients with Parkinson's disease. Exp Neurol. 1997;145:580–5. - PubMed
-
- Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

