A protocadherin-cadherin-FLRT3 complex controls cell adhesion and morphogenesis
- PMID: 20027292
- PMCID: PMC2791867
- DOI: 10.1371/journal.pone.0008411
A protocadherin-cadherin-FLRT3 complex controls cell adhesion and morphogenesis
Abstract
Background: Paraxial protocadherin (PAPC) and fibronectin leucine-rich domain transmembrane protein-3 (FLRT3) are induced by TGFbeta signaling in Xenopus embryos and both regulate morphogenesis by inhibiting C-cadherin mediated cell adhesion.
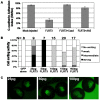
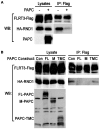
Principal findings: We have investigated the functional and physical relationships between PAPC, FLRT3, and C-cadherin. Although neither PAPC nor FLRT3 are required for each other to regulate C-cadherin adhesion, they do interact functionally and physically, and they form a complex with cadherins. By itself PAPC reduces cell adhesion physiologically to induce cell sorting, while FLRT3 disrupts adhesion excessively to cause cell dissociation. However, when expressed together PAPC limits the cell dissociating and tissue disrupting activity of FLRT3 to make it effective in physiological cell sorting. PAPC counteracts FLRT3 function by inhibiting the recruitment of the GTPase RND1 to the FLRT3 cytoplasmic domain.
Conclusions/significance: PAPC and FLRT3 form a functional complex with cadherins and PAPC functions as a molecular "governor" to maintain FLRT3 activity at the optimal level for physiological regulation of C-cadherin adhesion, cell sorting, and morphogenesis.
Conflict of interest statement
Figures




References
-
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–4690. - PubMed
-
- Wunnenberg-Stapleton K, Blitz IL, Hashimoto C, Cho KW. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development. 1999;126:5339–5351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

