rBPI(21) promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes
- PMID: 20027298
- PMCID: PMC2792722
- DOI: 10.1371/journal.pone.0008385
rBPI(21) promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes
Abstract
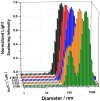
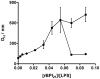
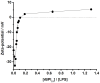
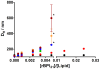
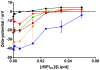
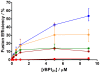
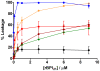
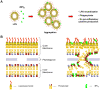
Antimicrobial peptides (AMPs) are important potential alternatives to conventional therapies against bacterial infections. rBPI(21) is a 21 kDa peptide based on the N-terminal region of the neutrophil bactericidal/permeability-increasing protein (BPI). This AMP possesses highly selective bactericidal effects on Gram-negative bacteria and have affinity for lipopolysaccharide (LPS) which is believed to be at the origin of its neutralizing effect of the LPS segregated into the bloodstream. We aim at understanding the molecular bases of rBPI(21) bactericidal and LPS neutralization actions, using biomembrane model systems. Using dynamic light scattering spectroscopy we demonstrate that rBPI(21) promotes aggregation of negatively charged large unilamellar vesicles (LUV), even in the absence of LPS, and LPS aggregates, while for zwitterionic phosphatidylcholine (POPC) LUV the size remains unchanged. The peptide also promotes the fusion (or hemifusion) of membranes containing phosphatidylglycerol (POPG). The aggregation and fusion of negatively charged LUV are peptide concentration-dependent until massive aggregation is reached, followed by sample flocculation/precipitation. Concomitantly, there is a progressive change in the zeta-potential of the LUV systems and LPS aggregates. LUV systems composed of phosphatidylglycerol (POPG) and lipid mixtures with POPG have higher zeta-potential variations than in the absence of POPG. The interaction of rBPI(21) with lipid vesicles is followed by leakage, with higher effect in POPG-containing membranes. LPS aggregation can be related with a decreased toxicity, possibly by facilitating its clearance by macrophage phagocytosis and/or blocking of LPS specific receptor recognition. Our data indicate that rBPI(21) mechanism of action at the molecular level involves the interaction with the LPS of the outer membrane of Gram-negative bacteria, followed by internalization and leakage induction through the (hemi)fusion of the bacterial outer and inner membranes, both enriched in phosphatidylglycerol.
Conflict of interest statement
Figures
Similar articles
-
Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems.Biopolymers. 2012;98(4):338-44. doi: 10.1002/bip.22095. Biopolymers. 2012. PMID: 23193598
-
rBPI21 interacts with negative membranes endothermically promoting the formation of rigid multilamellar structures.Biochim Biophys Acta. 2013 Nov;1828(11):2419-27. doi: 10.1016/j.bbamem.2013.06.009. Epub 2013 Jun 18. Biochim Biophys Acta. 2013. PMID: 23792068
-
Fold-unfold transitions in the selectivity and mechanism of action of the N-terminal fragment of the bactericidal/permeability-increasing protein (rBPI(21)).Biophys J. 2009 Feb;96(3):987-96. doi: 10.1016/j.bpj.2008.10.044. Biophys J. 2009. PMID: 19186136 Free PMC article.
-
Antimicrobial peptide rBPI21: a translational overview from bench to clinical studies.Curr Protein Pept Sci. 2012 Nov;13(7):611-9. doi: 10.2174/138920312804142101. Curr Protein Pept Sci. 2012. PMID: 23116442 Review.
-
The bactericidal/permeability increasing protein of neutrophils is a potent antibacterial and anti-endotoxin agent in vitro and in vivo.Prog Clin Biol Res. 1994;388:41-51. Prog Clin Biol Res. 1994. PMID: 7831373 Review.
Cited by
-
Endotoxin elimination in sepsis: physiology and therapeutic application.Langenbecks Arch Surg. 2010 Aug;395(6):597-605. doi: 10.1007/s00423-010-0658-6. Epub 2010 Jun 27. Langenbecks Arch Surg. 2010. PMID: 20582603 Review.
-
New Potent Membrane-Targeting Antibacterial Peptides from Viral Capsid Proteins.Front Microbiol. 2017 May 4;8:775. doi: 10.3389/fmicb.2017.00775. eCollection 2017. Front Microbiol. 2017. PMID: 28522994 Free PMC article.
-
The Effect of Lipopolysaccharides from Salmonella enterica on the Size, Density, and Compressibility of Phospholipid Vesicles.Biomimetics (Basel). 2025 Jan 15;10(1):55. doi: 10.3390/biomimetics10010055. Biomimetics (Basel). 2025. PMID: 39851772 Free PMC article.
-
Interaction of meso-tetrakis (4-N-methylpyridyl) porphyrin in its free base and as a Zn(II) derivative with large unilamellar phospholipid vesicles.Eur Biophys J. 2013 Apr;42(4):267-79. doi: 10.1007/s00249-012-0872-y. Epub 2012 Dec 12. Eur Biophys J. 2013. PMID: 23233118
-
Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins.J Virol. 2012 Feb;86(4):2096-108. doi: 10.1128/JVI.06796-11. Epub 2011 Nov 30. J Virol. 2012. PMID: 22130547 Free PMC article.
References
-
- Gura T. Innate immunity. Ancient system gets new respect. Science. 2001;291:2068–2071. - PubMed
-
- Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–547. - PubMed
-
- Dawson RM, Liu CQ. Properties and applications of antimicrobial peptides in biodefense against biological warfare threat agents. Crit Rev Microbiol. 2008;34:89–107. - PubMed
-
- Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

