Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis
- PMID: 20027303
- PMCID: PMC2793008
- DOI: 10.1371/journal.pone.0008359
Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis
Abstract
Background: The mechanism by which megakaryocytes (Mks) proliferate, differentiate, and release platelets into circulation are not well understood. Growing evidence indicates that a complex regulatory mechanism, involving cellular interactions, composition of the extracellular matrix and physical parameters such as oxygen tension, may contribute to the quiescent or permissive microenvironment related to Mk differentiation and maturation within the bone marrow.
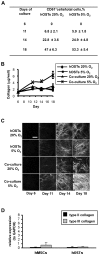
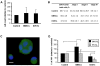
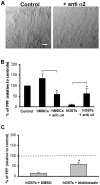
Methodology/principal findings: Differentiating human mesenchymal stem cells (hMSCs) into osteoblasts (hOSTs), we established an in vitro model for the osteoblastic niche. We demonstrated for the first time that the combination of HSCs, Mks and hypoxia sustain and promote bone formation by increasing type I collagen release from hOSTs and enhancing its fibrillar organization, as revealed by second harmonic generation microscopy. Through co-culture, we demonstrated that direct cell-cell contact modulates Mk maturation and differentiation. In particular we showed that low oxygen tension and direct interaction of hematopoietic stem cells (HSCs) with hOSTs inhibits Mk maturation and proplatelet formation (PPF). This regulatory mechanism was dependent on the fibrillar structure of type I collagen released by hOSTs and on the resulting engagement of the alpha2beta1 integrin. In contrast, normoxic conditions and the direct interaction of HSCs with undifferentiated hMSCs promoted Mk maturation and PPF, through a mechanism involving the VCAM-1 pathway.
Conclusions/significance: By combining cellular, physical and biochemical parameters, we mimicked an in vitro model of the osteoblastic niche that provides a physiological quiescent microenvironment where Mk differentiation and PPF are prevented. These findings serve as an important step in developing suitable in vitro systems to use for the study and manipulation of Mk differentiation and maturation in both normal and diseased states.
Conflict of interest statement
Figures
References
-
- Schulze H, Shivdasani RA. Mechanisms of thrombopoiesis. J Thromb Haemost. 2005;3:1717–1724. - PubMed
-
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. - PubMed
-
- Larson MK, Watson SP. A product of their environment: do megakaryocytes rely on extracellular cues for proplatelet formation? Platelets. 2006;17:435–440. - PubMed
-
- Leven RM, Tablin F. Extracellular matrix stimulation of guinea pig megakaryocyte proplatelet formation in vitro is mediated through the vitronectin receptor. Exp Hematol. 1992;20:1316–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

