Flotillins are involved in the polarization of primitive and mature hematopoietic cells
- PMID: 20027317
- PMCID: PMC2794375
- DOI: 10.1371/journal.pone.0008290
Flotillins are involved in the polarization of primitive and mature hematopoietic cells
Abstract
Background: Migration of mature and immature leukocytes in response to chemokines is not only essential during inflammation and host defense, but also during development of the hematopoietic system. Many molecules implicated in migratory polarity show uniform cellular distribution under non-activated conditions, but acquire a polarized localization upon exposure to migratory cues.
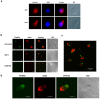
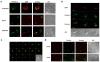
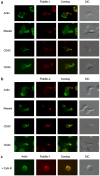
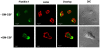
Methodology/principal findings: Here, we present evidence that raft-associated endocytic proteins (flotillins) are pre-assembled in lymphoid, myeloid and primitive hematopoietic cells and accumulate in the uropod during migration. Furthermore, flotillins display a polarized distribution during immunological synapse formation. Employing the membrane lipid-order sensitive probe Laurdan, we show that flotillin accumulation in the immunological synapse is concomittant with membrane ordering in these regions.
Conclusions: Together with the observation that flotillin polarization does not occur in other polarized cell types such as polarized epithelial cells, our results suggest a specific role for flotillins in hematopoietic cell polarization. Based on our results, we propose that in hematopoietic cells, flotillins provide intrinsic cues that govern segregation of certain microdomain-associated molecules during immune cell polarization.
Conflict of interest statement
Figures


Similar articles
-
Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod.PLoS One. 2009;4(4):e5403. doi: 10.1371/journal.pone.0005403. Epub 2009 Apr 30. PLoS One. 2009. PMID: 19404397 Free PMC article.
-
Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes.BMC Cell Biol. 2011 Jun 22;12:28. doi: 10.1186/1471-2121-12-28. BMC Cell Biol. 2011. PMID: 21696602 Free PMC article.
-
Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells.Blood. 2004 Oct 15;104(8):2332-8. doi: 10.1182/blood-2004-02-0511. Epub 2004 Jul 1. Blood. 2004. PMID: 15231568
-
Flotillins in membrane trafficking and physiopathology.Biol Cell. 2025 Jan;117(1):e2400134. doi: 10.1111/boc.202400134. Biol Cell. 2025. PMID: 39877933 Free PMC article. Review.
-
Flotillin membrane domains in cancer.Cancer Metastasis Rev. 2020 Jun;39(2):361-374. doi: 10.1007/s10555-020-09873-y. Cancer Metastasis Rev. 2020. PMID: 32297092 Free PMC article. Review.
Cited by
-
Pre-organized landscape of T cell surface.Front Immunol. 2023 Sep 13;14:1264721. doi: 10.3389/fimmu.2023.1264721. eCollection 2023. Front Immunol. 2023. PMID: 37795089 Free PMC article. Review.
-
Caveolin isoform switching as a molecular, structural, and metabolic regulator of microglia.Mol Cell Neurosci. 2013 Sep;56:283-97. doi: 10.1016/j.mcn.2013.07.002. Epub 2013 Jul 10. Mol Cell Neurosci. 2013. PMID: 23851187 Free PMC article.
-
Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment.J Cell Biol. 2010 Nov 15;191(4):771-81. doi: 10.1083/jcb.201005140. Epub 2010 Nov 8. J Cell Biol. 2010. PMID: 21059848 Free PMC article.
-
Endocytic trafficking of membrane-bound cargo: a flotillin point of view.Membranes (Basel). 2014 Jul 11;4(3):356-71. doi: 10.3390/membranes4030356. Membranes (Basel). 2014. PMID: 25019426 Free PMC article.
-
Primary human leukocyte subsets differentially express vaccinia virus receptors enriched in lipid rafts.J Virol. 2013 Aug;87(16):9301-12. doi: 10.1128/JVI.01545-13. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785200 Free PMC article.
References
-
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. - PubMed
-
- Serrador JM, Nieto M, Sanchez-Madrid F. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol. 1999;9:228–233. - PubMed
-
- Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. - PubMed
-
- Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91:4632–4644. - PubMed
-
- Dustin ML, Chan AC. Signaling takes shape in the immune system. Cell. 2000;103:283–294. - PubMed

