Mobile loop mutations in an archaeal inositol monophosphatase: modulating three-metal ion assisted catalysis and lithium inhibition
- PMID: 20027624
- PMCID: PMC2865715
- DOI: 10.1002/pro.315
Mobile loop mutations in an archaeal inositol monophosphatase: modulating three-metal ion assisted catalysis and lithium inhibition
Abstract
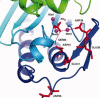
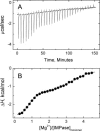
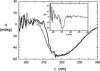
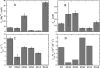
The inositol monophosphatase (IMPase) enzyme from the hyperthermophilic archaeon Methanocaldococcus jannaschii requires Mg(2+) for activity and binds three to four ions tightly in the absence of ligands: K(D) = 0.8 muM for one ion with a K(D) of 38 muM for the other Mg(2+) ions. However, the enzyme requires 5-10 mM Mg(2+) for optimum catalysis, suggesting substrate alters the metal ion affinity. In crystal structures of this archaeal IMPase with products, one of the three metal ions is coordinated by only one protein contact, Asp38. The importance of this and three other acidic residues in a mobile loop that approaches the active site was probed with mutational studies. Only D38A exhibited an increased kinetic K(D) for Mg(2+); D26A, E39A, and E41A showed no significant change in the Mg(2+) requirement for optimal activity. D38A also showed an increased K(m), but little effect on k(cat). This behavior is consistent with this side chain coordinating the third metal ion in the substrate complex, but with sufficient flexibility in the loop such that other acidic residues could position the Mg(2+) in the active site in the absence of Asp38. While lithium ion inhibition of the archaeal IMPase is very poor (IC(50) approximately 250 mM), the D38A enzyme has a dramatically enhanced sensitivity to Li(+) with an IC(50) of 12 mM. These results constitute additional evidence for three metal ion assisted catalysis with substrate and product binding reducing affinity of the third necessary metal ion. They also suggest a specific mode of action for lithium inhibition in the IMPase superfamily.
Figures

Similar articles
-
Purification and biochemical characterization of Mycobacterium tuberculosis SuhB, an inositol monophosphatase involved in inositol biosynthesis.Biochemistry. 2002 Apr 2;41(13):4392-8. doi: 10.1021/bi0160056. Biochemistry. 2002. PMID: 11914086
-
ONIOM (DFT:MM) study of the catalytic mechanism of myo-inositol monophosphatase: essential role of water in enzyme catalysis in the two-metal mechanism.J Phys Chem B. 2013 Jan 24;117(3):833-42. doi: 10.1021/jp312483n. Epub 2013 Jan 11. J Phys Chem B. 2013. PMID: 23268704
-
High-resolution structure of myo-inositol monophosphatase, the putative target of lithium therapy.Acta Crystallogr D Biol Crystallogr. 2005 May;61(Pt 5):545-55. doi: 10.1107/S0907444905004038. Epub 2005 Apr 20. Acta Crystallogr D Biol Crystallogr. 2005. PMID: 15858264
-
Structure and mechanism of inositol monophosphatase.FEBS Lett. 1995 Mar 13;361(1):1-7. doi: 10.1016/0014-5793(95)00063-f. FEBS Lett. 1995. PMID: 7890024 Review.
-
Inositol monophosphatase--a putative target for Li+ in the treatment of bipolar disorder.Trends Neurosci. 1995 Aug;18(8):343-9. doi: 10.1016/0166-2236(95)93926-o. Trends Neurosci. 1995. PMID: 7482796 Review.
Cited by
-
Transferable interactions of Li+ and Mg2+ ions in polarizable models.J Chem Phys. 2020 Sep 14;153(10):104113. doi: 10.1063/5.0022060. J Chem Phys. 2020. PMID: 32933310 Free PMC article.
-
ResidueFinder: extracting individual residue mentions from protein literature.J Biomed Semantics. 2021 Jul 21;12(1):14. doi: 10.1186/s13326-021-00243-3. J Biomed Semantics. 2021. PMID: 34289903 Free PMC article.
-
A Novel Phosphatase Reverses the Leloir Pathway to Promote Tagatose Synthesis from Glucose.bioRxiv [Preprint]. 2025 Aug 1:2025.07.29.665981. doi: 10.1101/2025.07.29.665981. bioRxiv. 2025. PMID: 40766469 Free PMC article. Preprint.
-
Systems Biology Understanding of the Effects of Lithium on Cancer.Front Oncol. 2019 Apr 30;9:296. doi: 10.3389/fonc.2019.00296. eCollection 2019. Front Oncol. 2019. PMID: 31114752 Free PMC article.
-
Cloning, overexpression, purification, crystallization and preliminary X-ray diffraction analysis of an inositol monophosphatase family protein (SAS2203) from Staphylococcus aureus MSSA476.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Apr 1;67(Pt 4):471-4. doi: 10.1107/S1744309111003496. Epub 2011 Mar 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21505244 Free PMC article.
References
-
- Majerus PW, Connolly TM, Bansal VS, Inhorn RC, Ross TS, Lips DL. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988;263:3051–3054. - PubMed
-
- Michell RH. Inositol derivatives: evolution and functions. Nat Rev Mol Cell Biol. 2008;9:151–161. - PubMed
-
- Scholz S, Sonnenbichler J, Schaefer W, Hensel R. Di-myo-inositol-1,1-phosphate in Pyrococcus woesei. FEMS Microbiol Lett. 1992;168:37–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

