MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells
- PMID: 20027628
- PMCID: PMC2891576
- DOI: 10.1002/ijc.25134
MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells
Abstract
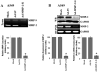
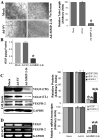
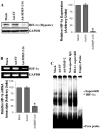
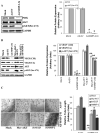
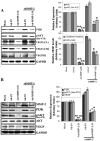
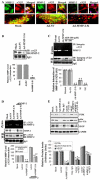
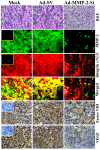
Vascular endothelial growth factor (VEGF) is one of the most important angiogenic growth factors for tumor angiogenesis. Here, we sought to explore whether RNA interference (RNAi) targeting matrix metalloproteinase-2 (MMP-2) could disrupt VEGF-mediated angiogenesis in lung cancer. MMP-2 siRNA inhibited lung cancer cell-induced tube formation of endothelial cells in vitro; addition of recombinant human-MMP-2 restored angiogenesis. MMP-2 transcriptional suppression decreased VEGF, phosphatidylinositol 3-kinase (PI3K) protein levels and AKT phosphorylation in lung cancer cells. In addition, MMP-2 suppression decreased hypoxia inducible factor-1alpha (HIF-1alpha), a transcription factor for VEGF, as determined by electrophoretic mobility shift assay (EMSA). We also show that MMP-2 suppression disrupted PI3K dependent VEGF expression; ectopic expression of myr-AKT restored VEGF inhibition. Further, MMP-2 suppression decreased the interaction of integrin-alphaVbeta3 and MMP-2 as confirmed by immunoprecipitation analyses. Studies with either function blocking integrin-alphaVbeta3 antibody or MMP-2 specific inhibitor (ARP-100) indicate that suppression of MMP-2 decreased integrin-alphaVbeta3-mediated induction of PI3K/AKT leading to decreased VEGF expression. Moreover, A549 xenograft tissue sections from mice that treated with MMP-2 siRNA showed reduced expression of VEGF and the angiogenic marker, factor-VIII. The inhibition of tumor angiogenesis in MMP-2 suppressed tumor sections was associated with decreased co-localization of integrin-alphaVbeta3 and MMP-2. In summary, these data provide new insights into the mechanisms underlying MMP-2-mediated VEGF expression in lung tumor angiogenesis.
Figures

Similar articles
-
Tissue inhibitor of metalloproteinase 3 suppresses tumor angiogenesis in matrix metalloproteinase 2-down-regulated lung cancer.Cancer Res. 2008 Jun 15;68(12):4736-45. doi: 10.1158/0008-5472.CAN-07-6612. Cancer Res. 2008. PMID: 18559520 Free PMC article.
-
Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis.Cardiovasc Res. 2008 May 1;78(2):356-65. doi: 10.1093/cvr/cvm111. Epub 2007 Dec 18. Cardiovasc Res. 2008. PMID: 18093986
-
Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells.Oncol Rep. 2017 Feb;37(2):777-784. doi: 10.3892/or.2016.5296. Epub 2016 Dec 7. Oncol Rep. 2017. PMID: 27959445
-
MMP-2 suppression abrogates irradiation-induced microtubule formation in endothelial cells by inhibiting αvβ3-mediated SDF-1/CXCR4 signaling.Int J Oncol. 2013 Apr;42(4):1279-88. doi: 10.3892/ijo.2013.1806. Epub 2013 Feb 4. Int J Oncol. 2013. PMID: 23381805 Free PMC article.
-
Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling.Mol Cell Biochem. 2013 Sep;381(1-2):267-72. doi: 10.1007/s11010-013-1710-y. Epub 2013 Jun 8. Mol Cell Biochem. 2013. PMID: 23749166
Cited by
-
Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity.Molecules. 2023 Jan 5;28(2):525. doi: 10.3390/molecules28020525. Molecules. 2023. PMID: 36677584 Free PMC article. Review.
-
Pyridine-Containing Macrocycles Display MMP-2/9 Inhibitory Activity and Distinct Effects on Migration and Invasion of 2D and 3D Breast Cancer Models.Int J Mol Sci. 2019 Oct 15;20(20):5109. doi: 10.3390/ijms20205109. Int J Mol Sci. 2019. PMID: 31618886 Free PMC article.
-
MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice.J Cell Sci. 2011 Aug 1;124(Pt 15):2666-75. doi: 10.1242/jcs.085480. Epub 2011 Jul 12. J Cell Sci. 2011. PMID: 21750188 Free PMC article.
-
MMP2 and MMP9 serum levels are associated with favorable outcome in patients with inflammatory breast cancer treated with bevacizumab-based neoadjuvant chemotherapy in the BEVERLY-2 study.Oncotarget. 2016 Apr 5;7(14):18531-40. doi: 10.18632/oncotarget.7612. Oncotarget. 2016. PMID: 26921265 Free PMC article. Clinical Trial.
-
Matrix metalloproteinase-2 cleavage of the β1 integrin ectodomain facilitates colon cancer cell motility.J Biol Chem. 2012 Oct 19;287(43):36556-66. doi: 10.1074/jbc.M112.384909. Epub 2012 Aug 16. J Biol Chem. 2012. PMID: 22898815 Free PMC article.
References
-
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. - PubMed
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. - PubMed
-
- McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Suppl 1):3–10. - PubMed
-
- Banerjee SN, Sengupta K, Banerjee S, Saxena NK, Banerjee SK. 2-Methoxyestradiol exhibits a biphasic effect on VEGF-A in tumor cells and upregulation is mediated through ER-alpha: a possible signaling pathway associated with the impact of 2-ME2 on proliferative cells. Neoplasia. 2003;5:417–26. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

