Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles
- PMID: 20028085
- PMCID: PMC2902264
- DOI: 10.1021/bc900438a
Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles
Abstract
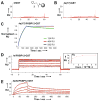
Nanoparticles bearing surface-conjugated targeting ligands are increasingly being explored for a variety of biomedical applications. The multivalent conjugation of targeting ligands on the surface of nanoparticles is presumed to enhance binding to the desired target. However, given the complexities inherent in the interactions of nanoparticle surfaces with proteins, and the structural diversity of nanoparticle scaffolds and targeting ligands, our understanding of how conjugation of targeting ligands affects nanoparticle binding remains incomplete. Here, we use surface plasmon resonance (SPR) to directly and quantitatively study the affinity and binding kinetics of nanoparticles that display small molecules conjugated to their surface. We studied the interaction between a single protein target and a structurally related series of targeting ligands whose intrinsic affinity varies over a 4500-fold range and performed SPR at protein densities that reflect endogenous receptor densities. We report that even weak small molecule targeting ligands can significantly enhance target-specific avidity (by up to 4 orders of magnitude) through multivalent interactions and also observe a much broader range of kinetic effects than has been previously reported. Quantitative measurement of how the affinity and kinetics of nanoparticle binding vary as a function of different surface conjugations is a rapid, generalizable approach to nanoparticle characterization that can inform the design of nanoparticles for biomedical applications.
Figures



References
-
- De M, Ghosh PS, Rotello VM. Applications of nanoparticles in biology. Adv Mater. 2008;20:4225–4241.
-
- Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. J Med Chem. 2006;49:6087–6093. - PubMed
-
- Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. - PubMed
-
- Mulder A, Huskens J, Reinhoudt DN. Multivalency in supramolecular chemistry and nanofabrication. Org Biomol Chem. 2004;2:3409–3424. - PubMed
-
- Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

