Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats
- PMID: 20028350
- PMCID: PMC2858236
- DOI: 10.1111/j.1530-0277.2009.01119.x
Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats
Abstract
Background: The purpose of this study was to re-examine intragastric ethanol intubation as a dependence induction method that effectively induces physical dependence upon ethanol over a short time period, is devoid of intrinsic stress artifacts, inexpensive, and easy to implement.
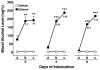
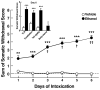
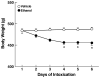
Methods: Male Wistar rats were subjected to ethanol dependence induction via intragastric ethanol intubation. Ethanol solution (final concentration 20%, made up in a dietary liquid vehicle consisting of powdered milk, sucrose, and water) was intubated 4 times per day, at 4-hour intervals, for 6 consecutive days (for a total of 10 g/kg/day). The utility of this procedure was evaluated for inducing physical dependence, determined by daily and final withdrawal ratings. Anxiety-like behavior associated with ethanol dependence history was examined using the elevated plus-maze (EPM) test, conducted 5 days after ethanol withdrawal. To evaluate whether potential stress-like effects of intragastric intubation per se produce lasting effects on behavior, experimentally naive rats were compared with vehicle-intubated rats for anxiety-like behavior on the EPM.
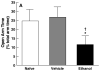
Results: Blood alcohol levels reached stable levels between 200 and 250 mg%, measured 1 hour after the second and third ethanol intubation on days 2, 4, and 6. Ethanol-treated rats developed significant somatic withdrawal signs, recorded daily between 10 and 12 hours after the last ethanol administration. At 5 days postwithdrawal, ethanol-treated rats showed significant anxiety-like behavior, measured by decreased open arm time and open arm entries on the EPM, compared with vehicle controls. Additionally, ethanol postdependent rats showed decreased open arm time compared with experimentally naive rats. EPM performance did not differ between vehicle-intubated and naive rats. No withdrawal seizures were observed and mortality rate was near zero.
Conclusions: These findings suggest that intragastric ethanol administration produces a behavioral profile consistent with ethanol dependence (i.e., significant withdrawal signs after termination of ethanol exposure and elevated anxiety-like behavior persisting beyond completion of physical withdrawal), and that the intubation procedure itself does not produce lasting nonspecific anxiety-like effects. Thus, under the conditions employed here, this procedure provides an effective tool for inducing and evaluating the consequences of ethanol dependence in animal models of ethanol reward and motivation.
Figures

References
-
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. - PubMed
-
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. - PubMed
-
- Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. - PubMed
-
- Feng HJ, Yang L, Faingold CL. Role of the amygdala in ethanol withdrawal seizures. Brain Res. 2007;1141:65–73. - PubMed
-
- File SE, Andrews N, Al-Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: effects of tianeptine. Alcohol Alcohol. 1993;28:281–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

