Plant sphingolipids: decoding the enigma of the Sphinx
- PMID: 20028469
- PMCID: PMC2848707
- DOI: 10.1111/j.1469-8137.2009.03123.x
Plant sphingolipids: decoding the enigma of the Sphinx
Abstract
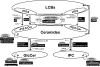
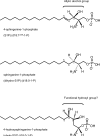
Sphingolipids are a ubiquitous class of lipids present in a variety of organisms including eukaryotes and bacteria. In the last two decades, research has focused on characterizing the individual species of this complex family of lipids, which has led to a new field of research called 'sphingolipidomics'. There are at least 500 (and perhaps thousands of) different molecular species of sphingolipids in cells, and in Arabidopsis alone it has been reported that there are at least 168 different sphingolipids. Plant sphingolipids can be divided into four classes: glycosyl inositol phosphoceramides (GIPCs), glycosylceramides, ceramides, and free long-chain bases (LCBs). Numerous enzymes involved in plant sphingolipid metabolism have now been cloned and characterized, and, in general, there is broad conservation in the way in which sphingolipids are metabolized in animals, yeast and plants. Here, we review the diversity of sphingolipids reported in the literature, some of the recent advances in our understanding of sphingolipid metabolism in plants, and the physiological roles that sphingolipids and sphingolipid metabolites play in plant physiology.
Figures



Similar articles
-
Sphingolipid metabolism, transport, and functions in plants: Recent progress and future perspectives.Plant Commun. 2021 Jun 29;2(5):100214. doi: 10.1016/j.xplc.2021.100214. eCollection 2021 Sep 13. Plant Commun. 2021. PMID: 34746760 Free PMC article. Review.
-
Diversity in sphingolipid metabolism across land plants.J Exp Bot. 2022 May 13;73(9):2785-2798. doi: 10.1093/jxb/erab558. J Exp Bot. 2022. PMID: 35560193 Free PMC article. Review.
-
GIPC: Glycosyl Inositol Phospho Ceramides, the major sphingolipids on earth.Plant Signal Behav. 2016;11(4):e1152438. doi: 10.1080/15592324.2016.1152438. Plant Signal Behav. 2016. PMID: 27074617 Free PMC article. Review.
-
Plant sphingolipids: function follows form.Curr Opin Plant Biol. 2013 Jun;16(3):350-7. doi: 10.1016/j.pbi.2013.02.009. Epub 2013 Mar 14. Curr Opin Plant Biol. 2013. PMID: 23499054 Review.
-
Separation and identification of major plant sphingolipid classes from leaves.J Biol Chem. 2006 Aug 11;281(32):22684-94. doi: 10.1074/jbc.M604050200. Epub 2006 Jun 12. J Biol Chem. 2006. PMID: 16772288
Cited by
-
Specific membrane lipid composition is important for plasmodesmata function in Arabidopsis.Plant Cell. 2015 Apr;27(4):1228-50. doi: 10.1105/tpc.114.135731. Epub 2015 Mar 27. Plant Cell. 2015. PMID: 25818623 Free PMC article.
-
Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses.Plant Physiol. 2012 Jul;159(3):1138-48. doi: 10.1104/pp.112.199547. Epub 2012 May 25. Plant Physiol. 2012. PMID: 22635113 Free PMC article.
-
Organization and dynamics of functional plant membrane microdomains.Cell Mol Life Sci. 2020 Jan;77(2):275-287. doi: 10.1007/s00018-019-03270-7. Epub 2019 Aug 17. Cell Mol Life Sci. 2020. PMID: 31422442 Free PMC article. Review.
-
Sweet Modifications Modulate Plant Development.Biomolecules. 2021 May 18;11(5):756. doi: 10.3390/biom11050756. Biomolecules. 2021. PMID: 34070047 Free PMC article. Review.
-
Extraction Optimization, UHPLC-Triple-TOF-MS/MS Analysis and Antioxidant Activity of Ceramides from Sea Red Rice Bran.Foods. 2022 May 12;11(10):1399. doi: 10.3390/foods11101399. Foods. 2022. PMID: 35626968 Free PMC article.
References
-
- Bartke N, Fischbeck A, Humpf HU. Analysis of sphingolipids in potatoes (Solanum tuberosum L.) and sweet potatoes (Ipomoea batatas (L.) Lam.) by reversed phase high-performance liquid chromatography electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) Molecular Nutition and Food Research. 2006;50:1201–1211. - PubMed
-
- Bhat RA, Panstruga R. Lipid rafts in plants. Planta. 2005;223:5–19. - PubMed
-
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. - PubMed
-
- Bille J, Weiser T, Bentrup F-W. The lysolipid sphingosine modulates pyrophosphatase activity in tonoplast vesicles and isolated vacuoles from a heterotrophic cell suspension culture of Chenopodium rubrum. Physiologia Plantarum. 1992;84:250–254.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

