Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms
- PMID: 20028487
- PMCID: PMC2937183
- DOI: 10.1111/j.1600-0854.2009.01029.x
Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms
Abstract
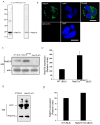
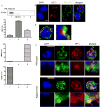
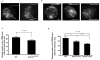
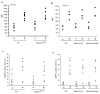
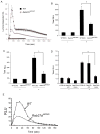
Neutrophils rely on exocytosis to mobilize receptors and adhesion molecules and to release microbicidal factors. This process should be strictly regulated because uncontrolled release of toxic proteins would be injurious to the host. In vivo studies showed that the small GTPase Rab27a regulates azurophilic granule exocytosis. Using mouse neutrophils deficient in Rab27a (Rab27a(ash/ash)), Rab27b [Rab27b knockout (KO)] or both [Rab27a/b double KO (DoKo)], we investigated the role of the Rab27 isoforms in neutrophils. We found that both Rab27a and Rab27b deficiencies impaired azurophilic granule exocytosis. Rab27a(ash/ash) neutrophils showed upregulation of Rab27b expression which did not compensate for the secretory defects observed in Rab27a-deficient cells, suggesting that Rab27 isoforms play independent roles in neutrophil exocytosis. Total internal reflection fluorescence microscopy analysis showed that Rab27a(ash/ash) and Rab27b KO neutrophils have a decreased number of azurophilic granules near the plasma membrane. The effect was exacerbated in Rab27a/b DoKo neutrophils. Rab27-deficient neutrophils showed impaired activation of the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase at the plasma membrane although intraphagosomal reactive oxygen species (ROS) production was not affected. Exocytosis of secretory vesicles in Rab27-deficient neutrophils was functional, suggesting that Rab27 GTPases selectively control the exocytosis of neutrophil granules.
Figures


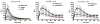
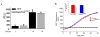
Similar articles
-
Identification of Neutrophil Exocytosis Inhibitors (Nexinhibs), Small Molecule Inhibitors of Neutrophil Exocytosis and Inflammation: DRUGGABILITY OF THE SMALL GTPase Rab27a.J Biol Chem. 2016 Dec 9;291(50):25965-25982. doi: 10.1074/jbc.M116.741884. Epub 2016 Oct 4. J Biol Chem. 2016. PMID: 27702998 Free PMC article.
-
Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion.FEBS J. 2013 Feb;280(3):892-903. doi: 10.1111/febs.12081. Epub 2013 Jan 2. FEBS J. 2013. PMID: 23281710
-
Munc13-4 restricts motility of Rab27a-expressing vesicles to facilitate lipopolysaccharide-induced priming of exocytosis in neutrophils.J Biol Chem. 2011 Feb 18;286(7):5647-56. doi: 10.1074/jbc.M110.184762. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148308 Free PMC article.
-
In vivo Roles of Rab27 and Its Effectors in Exocytosis.Cell Struct Funct. 2021 Nov 6;46(2):79-94. doi: 10.1247/csf.21043. Epub 2021 Sep 4. Cell Struct Funct. 2021. PMID: 34483204 Free PMC article. Review.
-
Rab27 effectors, pleiotropic regulators in secretory pathways.Traffic. 2013 Sep;14(9):949-63. doi: 10.1111/tra.12083. Epub 2013 Jun 10. Traffic. 2013. PMID: 23678941 Review.
Cited by
-
Neutrophil azurophilic granule exocytosis is primed by TNF-α and partially regulated by NADPH oxidase.Innate Immun. 2016 Nov;22(8):635-646. doi: 10.1177/1753425916668980. Epub 2016 Sep 23. Innate Immun. 2016. PMID: 27655046 Free PMC article.
-
Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli.J Immunol. 2017 Nov 15;199(10):3559-3570. doi: 10.4049/jimmunol.1700904. Epub 2017 Oct 4. J Immunol. 2017. PMID: 28978688 Free PMC article.
-
MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane.J Clin Invest. 2016 Nov 1;126(11):4125-4139. doi: 10.1172/JCI87043. Epub 2016 Oct 4. J Clin Invest. 2016. PMID: 27701149 Free PMC article.
-
Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells.PLoS One. 2010 Sep 21;5(9):e12870. doi: 10.1371/journal.pone.0012870. PLoS One. 2010. PMID: 20877725 Free PMC article.
-
Rab27a Regulates Human Perivascular Adipose Progenitor Cell Differentiation.Cardiovasc Drugs Ther. 2018 Oct;32(5):519-530. doi: 10.1007/s10557-018-6813-y. Cardiovasc Drugs Ther. 2018. PMID: 30105417 Free PMC article.
References
-
- Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Bainton DF. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. - PubMed
-
- Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. - PMC - PubMed
-
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. - PMC - PubMed
-
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

