Amyloid and tau cerebrospinal fluid biomarkers in HIV infection
- PMID: 20028512
- PMCID: PMC2807422
- DOI: 10.1186/1471-2377-9-63
Amyloid and tau cerebrospinal fluid biomarkers in HIV infection
Abstract
Background: Because of the emerging intersections of HIV infection and Alzheimer's disease, we examined cerebrospinal fluid (CSF) biomarkers related of amyloid and tau metabolism in HIV-infected patients.
Methods: In this cross-sectional study we measured soluble amyloid precursor proteins alpha and beta (sAPPalpha and sAPPbeta), amyloid beta fragment 1-42 (Abeta1-42), and total and hyperphosphorylated tau (t-tau and p-tau) in CSF of 86 HIV-infected (HIV+) subjects, including 21 with AIDS dementia complex (ADC), 25 with central nervous system (CNS) opportunistic infections and 40 without neurological symptoms and signs. We also measured these CSF biomarkers in 64 uninfected (HIV-) subjects, including 21 with Alzheimer's disease, and both younger and older controls without neurological disease.
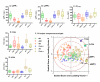
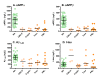
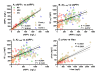
Results: CSF sAPPalpha and sAPPbeta concentrations were highly correlated and reduced in patients with ADC and opportunistic infections compared to the other groups. The opportunistic infection group but not the ADC patients had lower CSF Abeta1-42 in comparison to the other HIV+ subjects. CSF t-tau levels were high in some ADC patients, but did not differ significantly from the HIV+ neuroasymptomatic group, while CSF p-tau was not increased in any of the HIV+ groups. Together, CSF amyloid and tau markers segregated the ADC patients from both HIV+ and HIV- neuroasymptomatics and from Alzheimer's disease patients, but not from those with opportunistic infections.
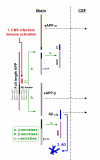
Conclusions: Parallel reductions of CSF sAPPalpha and sAPPbeta in ADC and CNS opportunistic infections suggest an effect of CNS immune activation or inflammation on neuronal amyloid synthesis or processing. Elevation of CSF t-tau in some ADC and CNS infection patients without concomitant increase in p-tau indicates neural injury without preferential accumulation of hyperphosphorylated tau as found in Alzheimer's disease. These biomarker changes define pathogenetic pathways to brain injury in ADC that differ from those of Alzheimer's disease.
Figures
References
-
- Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, Abramson I, Atkinson JH, Grant I, McCutchan JA. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42(5):679–688. doi: 10.1002/ana.410420503. - DOI - PubMed
-
- Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21(4):271–276. - PubMed
-
- Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158(5):1079–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

