Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family
- PMID: 20028514
- PMCID: PMC2805614
- DOI: 10.1186/1471-2121-10-95
Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family
Abstract
Background: Tight junctions are an intercellular adhesion complex of epithelial and endothelial cells, and form a paracellular barrier that restricts the diffusion of solutes on the basis of size and charge. Tight junctions are formed by multiprotein complexes containing cytosolic and transmembrane proteins. How these components work together to form functional tight junctions is still not well understood and will require a complete understanding of the molecular composition of the junction.
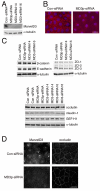
Results: Here we identify a new transmembrane component of tight junctions: MarvelD3, a four-span transmembrane protein. Its predicted transmembrane helices form a Marvel (MAL and related proteins for vesicle traffic and membrane link) domain, a structural motif originally discovered in proteins involved in membrane apposition and fusion events, such as the tight junction proteins occludin and tricellulin. In mammals, MarvelD3 is expressed as two alternatively spliced isoforms. Both isoforms exhibit a broad tissue distribution and are expressed by different types of epithelial as well as endothelial cells. MarvelD3 co-localises with occludin at tight junctions in intestinal and corneal epithelial cells. RNA interference experiments in Caco-2 cells indicate that normal MarvelD3 expression is not required for the formation of functional tight junctions but depletion results in monolayers with increased transepithelial electrical resistance.
Conclusions: Our data indicate that MarvelD3 is a third member of the tight junction-associated occludin family of transmembrane proteins. Similar to occludin, normal expression of MarvelD3 is not essential for the formation of functional tight junctions. However, MarvelD3 functions as a determinant of epithelial paracellular permeability properties.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

