Cellular adaptive immune response against porcine circovirus type 2 in subclinically infected pigs
- PMID: 20028550
- PMCID: PMC2806361
- DOI: 10.1186/1746-6148-5-45
Cellular adaptive immune response against porcine circovirus type 2 in subclinically infected pigs
Abstract
Background: Porcine circovirus type 2 (PCV2) is a dominant causative agent of postweaning multisystemic wasting syndrome (PMWS), a multifactorial disease complex with putative immunosuppressive characteristics. Little is known about adaptive PCV2-specific immune responses in infected pigs. Therefore, the T and B cell responses following PCV2 infection in 3-week old SPF piglets infected with PCV2 or PCV2 plus porcine parvovirus (PPV) were studied.
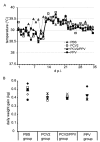
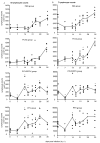
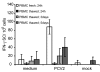
Results: All animals were asymptomatically infected. At 7 days post infection (d p.i.), B lymphocyte and T lymphocyte numbers decreased in the dual infected, but not in the single infected piglets. At this time point a transient PCV2 viraemia was noted in the PCV2 infected groups. Antibodies against the infecting virus were detectable at day 24-28 p.i. for anti-PCV2 antibodies and at day 10 p.i. for anti-PPV antibodies, with no apparent influence of PCV2 on the early PPV antibody development. In the animals infected with PPV alone, IFN-gamma secreting cells (SC) that were not specific for PCV2 were detected by ELISPOT assay at day 7 p.i. Interestingly, this response was absent in the PCV2/PPV dual infected animals. PCV2-specific IFN-gamma SC were observed in the PCV2/PPV infected group at 7 d p.i. and in the PCV2 single infected group at 21 d p.i. A reduction in the numbers of IFN-gamma SC was observed following anti-CD4 and anti-CD8 antibody treatment, suggesting roles for both CD4+ and CD8+ T cells in the response against PCV2 infection. This was supported by an observed increase in the percentage of IFN-gamma positive CD8hi cytotoxic T cells as well as IFN-gamma positive CD8-/low helper T cells after PCV2 in vitro re-stimulation.
Conclusions: Infection of weaned SPF piglets with PCV2 alone or combined with PPV does not induce disease and in both cases a relatively slow anti-PCV2 antibody response and weak T lymphocyte responses were found. Knowledge on such immunological characteristics is important for both PCV2 pathogenesis and vaccination.
Figures
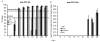

Similar articles
-
Markedly different immune responses and virus kinetics in littermates infected with porcine circovirus type 2 or porcine parvovirus type 1.Vet Immunol Immunopathol. 2017 Sep;191:51-59. doi: 10.1016/j.vetimm.2017.08.003. Epub 2017 Aug 9. Vet Immunol Immunopathol. 2017. PMID: 28895867
-
Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus.Vet Microbiol. 2004 Mar 5;98(3-4):209-20. doi: 10.1016/j.vetmic.2003.11.006. Vet Microbiol. 2004. PMID: 15036529
-
Comparative effects of vaccination against porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) in a PCV2-PRRSV challenge model.Clin Vaccine Immunol. 2013 Mar;20(3):369-76. doi: 10.1128/CVI.00497-12. Epub 2013 Jan 9. Clin Vaccine Immunol. 2013. PMID: 23302743 Free PMC article.
-
Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system.Annu Rev Anim Biosci. 2013 Jan;1:43-64. doi: 10.1146/annurev-animal-031412-103720. Epub 2013 Jan 3. Annu Rev Anim Biosci. 2013. PMID: 25387012 Review.
-
Immunology of porcine circovirus type 2 (PCV2).Virus Res. 2012 Mar;164(1-2):61-7. doi: 10.1016/j.virusres.2011.12.003. Epub 2011 Dec 9. Virus Res. 2012. PMID: 22178803 Review.
Cited by
-
Cellular immune signatures and differences of four porcine circovirus type 2 vaccines to heterologous PCV2d infection.NPJ Vaccines. 2025 May 10;10(1):92. doi: 10.1038/s41541-025-01138-5. NPJ Vaccines. 2025. PMID: 40348755 Free PMC article.
-
Impact of maternally derived immunity on piglets' immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCV2 at different age.BMC Vet Res. 2016 May 11;12:77. doi: 10.1186/s12917-016-0700-1. BMC Vet Res. 2016. PMID: 27170186 Free PMC article. Clinical Trial.
-
Suppressor of cytokine signaling 3 plays an important role in porcine circovirus type 2 subclinical infection by downregulating proinflammatory responses.Sci Rep. 2016 Sep 1;6:32538. doi: 10.1038/srep32538. Sci Rep. 2016. PMID: 27581515 Free PMC article.
-
PCV2 vaccination induces IFN-γ/TNF-α co-producing T cells with a potential role in protection.Vet Res. 2015 Mar 3;46:20. doi: 10.1186/s13567-015-0157-4. Vet Res. 2015. PMID: 25888899 Free PMC article.
-
Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2.Vaccines (Basel). 2025 Jul 8;13(7):736. doi: 10.3390/vaccines13070736. Vaccines (Basel). 2025. PMID: 40733713 Free PMC article.
References
-
- Todd D, Bendinelli M, Biagini P, Hino S, Mankertz A, Mishiro S, Niel C, Okamoto H, Raidal S, Ritchie BW, Teo GC. In: Virus taxonomy: Eight report of the international committee on taxonomy of viruses. 2. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editor. San Diego: Elsevier academic press; 2005. Circoviridae; pp. 327–334.
-
- Allan GM, McNeilly F, Meehan BM, Kennedy S, Mackie DP, Ellis JA, Clark EG, Espuna E, Saubi N, Riera P. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet Microbiol. 1999;66(2):115–123. doi: 10.1016/S0378-1135(99)00004-8. - DOI - PubMed
-
- Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG, Ellis JA, Haines DM, Meehan BM, Adair BM. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998;10(1):3–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

