Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression
- PMID: 20028558
- PMCID: PMC2803201
- DOI: 10.1186/1471-2164-10-625
Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression
Abstract
Background: Pulmonary metastasis continues to be the most common cause of death in osteosarcoma. Indeed, the 5-year survival for newly diagnosed osteosarcoma patients has not significantly changed in over 20 years. Further understanding of the mechanisms of metastasis and resistance for this aggressive pediatric cancer is necessary. Pet dogs naturally develop osteosarcoma providing a novel opportunity to model metastasis development and progression. Given the accelerated biology of canine osteosarcoma, we hypothesized that a direct comparison of canine and pediatric osteosarcoma expression profiles may help identify novel metastasis-associated tumor targets that have been missed through the study of the human cancer alone.
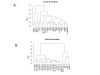
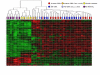
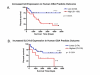
Results: Using parallel oligonucleotide array platforms, shared orthologues between species were identified and normalized. The osteosarcoma expression signatures could not distinguish the canine and human diseases by hierarchical clustering. Cross-species target mining identified two genes, interleukin-8 (IL-8) and solute carrier family 1 (glial high affinity glutamate transporter), member 3 (SLC1A3), which were uniformly expressed in dog but not in all pediatric osteosarcoma patient samples. Expression of these genes in an independent population of pediatric osteosarcoma patients was associated with poor outcome (p = 0.020 and p = 0.026, respectively). Validation of IL-8 and SLC1A3 protein expression in pediatric osteosarcoma tissues further supported the potential value of these novel targets. Ongoing evaluation will validate the biological significance of these targets and their associated pathways.
Conclusions: Collectively, these data support the strong similarities between human and canine osteosarcoma and underline the opportunities provided by a comparative oncology approach as a means to improve our understanding of cancer biology and therapies.
Figures

References
-
- Varan A, Yazici N, Aksoy C, Gedikoglu G, Yalcin B, Akyuz C, Kutluk T, Buyukpamukcu M. Treatment results of pediatric osteosarcoma: twenty-year experience. J Pediatr Orthop. 2007;27(2):241–246. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

