The Pseudomonas aeruginosa magnesium transporter MgtE inhibits transcription of the type III secretion system
- PMID: 20028803
- PMCID: PMC2825933
- DOI: 10.1128/IAI.00865-09
The Pseudomonas aeruginosa magnesium transporter MgtE inhibits transcription of the type III secretion system
Abstract
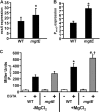
Pseudomonas aeruginosa is an opportunistic pathogen that causes life-long pneumonia in individuals with cystic fibrosis (CF). These long-term infections are maintained by bacterial biofilm formation in the CF lung. We have recently developed a model of P. aeruginosa biofilm formation on cultured CF airway epithelial cells. Using this model, we discovered that mutation of a putative magnesium transporter gene, called mgtE, led to increased cytotoxicity of P. aeruginosa toward epithelial cells. This altered toxicity appeared to be dependent upon expression of the type III secretion system (T3SS). In this study, we found that mutation of mgtE results in increased T3SS gene transcription. Through epistasis analyses, we discovered that MgtE influences the ExsE-ExsC-ExsD-ExsA gene regulatory system of T3SS by either directly or indirectly inhibiting ExsA activity. While variations in calcium levels modulate T3SS gene expression in P. aeruginosa, we found that addition of exogenous magnesium did not inhibit T3SS activity. Furthermore, mgtE variants that were defective for magnesium transport could still complement the cytotoxicity effect. Thus, the magnesium transport function of MgtE does not fully explain the regulatory effects of MgtE on cytotoxicity. Overall, our results indicate that MgtE modulates expression of T3SS genes.
Figures

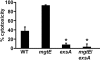



Similar articles
-
Pseudomonas aeruginosa Magnesium Transporter MgtE Inhibits Type III Secretion System Gene Expression by Stimulating rsmYZ Transcription.J Bacteriol. 2017 Oct 31;199(23):e00268-17. doi: 10.1128/JB.00268-17. Print 2017 Dec 1. J Bacteriol. 2017. PMID: 28847924 Free PMC article.
-
Antibiotic treatment of Pseudomonas aeruginosa biofilms stimulates expression of the magnesium transporter gene mgtE.Microbiology (Reading). 2014 Jan;160(Pt 1):165-178. doi: 10.1099/mic.0.070144-0. Epub 2013 Oct 25. Microbiology (Reading). 2014. PMID: 24162608
-
MgtE is a dual-function protein in Pseudomonas aeruginosa.Microbiology (Reading). 2014 Jun;160(Pt 6):1200-1213. doi: 10.1099/mic.0.075275-0. Epub 2014 Apr 10. Microbiology (Reading). 2014. PMID: 24722909
-
Fitting Pieces into the Puzzle of Pseudomonas aeruginosa Type III Secretion System Gene Expression.J Bacteriol. 2019 Jun 10;201(13):e00209-19. doi: 10.1128/JB.00209-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 31010903 Free PMC article. Review.
-
Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system.Mol Microbiol. 2006 Nov;62(3):631-40. doi: 10.1111/j.1365-2958.2006.05412.x. Epub 2006 Sep 21. Mol Microbiol. 2006. PMID: 16995895 Review.
Cited by
-
Mycoplasma non-coding RNA: identification of small RNAs and targets.BMC Genomics. 2016 Oct 25;17(Suppl 8):743. doi: 10.1186/s12864-016-3061-z. BMC Genomics. 2016. PMID: 27801290 Free PMC article.
-
Acidosis potentiates the host proinflammatory interleukin-1β response to Pseudomonas aeruginosa infection.Infect Immun. 2014 Nov;82(11):4689-97. doi: 10.1128/IAI.02024-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156732 Free PMC article.
-
Impact of alginate-producing Pseudomonas aeruginosa on alveolar macrophage apoptotic cell clearance.J Cyst Fibros. 2015 Jan;14(1):70-77. doi: 10.1016/j.jcf.2014.06.009. Epub 2014 Jul 12. J Cyst Fibros. 2015. PMID: 25027418 Free PMC article.
-
DNA alternate polymerase PolB mediates inhibition of type III secretion in Pseudomonas aeruginosa.Microbes Infect. 2021 Mar-Apr;23(2-3):104777. doi: 10.1016/j.micinf.2020.11.004. Epub 2020 Dec 1. Microbes Infect. 2021. PMID: 33276123 Free PMC article.
-
Fis Regulates Type III Secretion System by Influencing the Transcription of exsA in Pseudomonas aeruginosa Strain PA14.Front Microbiol. 2017 Apr 19;8:669. doi: 10.3389/fmicb.2017.00669. eCollection 2017. Front Microbiol. 2017. PMID: 28469612 Free PMC article.
References
-
- Boucher, R. C. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146-158. - PubMed
-
- Bruscia, E., F. Sangiuolo, P. Sinibaldi, K. K. Goncz, G. Novelli, and D. C. Gruenert. 2002. Isolation of CF cell lines corrected at ΔF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 9:683-685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

