Pharmacokinetic-pharmacodynamic modeling of alpha interferon response induced by a Toll-like 7 receptor agonist in mice
- PMID: 20028817
- PMCID: PMC2825998
- DOI: 10.1128/AAC.00551-09
Pharmacokinetic-pharmacodynamic modeling of alpha interferon response induced by a Toll-like 7 receptor agonist in mice
Abstract
Recombinant alpha interferon (IFN-alpha) is used in the treatment of hepatitis C virus (HCV)-infected patients but is not optimal in terms of efficacy or tolerability. Toll-like 7 receptor (TLR-7) agonists stimulate the innate immune system to produce, among other cytokines, IFN-alpha and are being evaluated as alternative drugs to treat HCV infection. This paper describes the application of pharmacokinetic-pharmacodynamic (PK-PD) modeling to understanding the behavior of a TLR-7 agonist [9-benzyl-8-hydroxy-2-(2-methoxyethoxy) adenine (BHMA)] in mice, using IFN-alpha as a biomarker. This is the first report of such a PK-PD model, and the conclusions may be of utility in the clinical development of TLR-7 agonists for HCV infection.
Figures
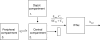

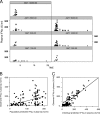
References
-
- Arany, I., S. K. Tyring, M. A. Stanley, M. A. Tomai, R. L. Miller, M. H. Smith, D. J. McDermott, and H. B. Slade. 1999. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5%. Antiviral Res. 43:55-63. - PubMed
-
- Averett, D. R., S. P. Fletcher, W. Li, S. E. Webber, and J. R. Appleman. 2007. The pharmacology of endosomal TLR agonists in viral disease. Biochem. Soc. Trans. 35:1468-1472. - PubMed
-
- Beal, S. L., and L. B. Sheiner. 1999. NONMEM user's guide. University of California at San Francisco, San Francisco.
-
- Bekkering, F. C., J. T. Brouwer, B. E. Hansen, and S. W. Schalm. 2001. Hepatitis C viral kinetics in difficult to treat patients receiving high dose interferon and ribavirin. J. Hepatol. 34:435-440. - PubMed
-
- Danhof, M., E. C. de Lange, O. E. Della Pasqua, B. A. Ploeger, and R. A. Voskuyl. 2008. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 29:186-191. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

