Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations
- PMID: 20028819
- PMCID: PMC2825970
- DOI: 10.1128/AAC.00963-09
Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations
Abstract
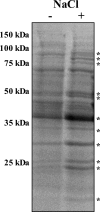
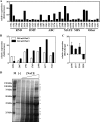
Acinetobacter baumannii is well adapted to the hospital environment, where infections caused by this organism are associated with significant morbidity and mortality. Genetic determinants of antimicrobial resistance have been described extensively, yet the mechanisms by which A. baumannii regulates antibiotic resistance have not been defined. We sought to identify signals encountered within the hospital setting or human host that alter the resistance phenotype of A. baumannii. In this regard, we have identified NaCl as being an important signal that induces significant tolerance to aminoglycosides, carbapenems, quinolones, and colistin upon the culturing of A. baumannii cells in physiological NaCl concentrations. Proteomic analyses of A. baumannii culture supernatants revealed the release of outer membrane proteins in high NaCl, including two porins (CarO and a 33- to 36-kDa protein) whose loss or inactivation is associated with antibiotic resistance. To determine if NaCl affected expression at the transcriptional level, the transcriptional response to NaCl was determined by microarray analyses. These analyses highlighted 18 genes encoding putative efflux transporters that are significantly upregulated in response to NaCl. Consistent with this, the effect of NaCl on the tolerance to levofloxacin and amikacin was significantly reduced upon the treatment of A. baumannii with an efflux pump inhibitor. The effect of physiological concentrations of NaCl on colistin resistance was conserved in a panel of multidrug-resistant isolates of A. baumannii, underscoring the clinical significance of these observations. Taken together, these data demonstrate that A. baumannii sets in motion a global regulatory cascade in response to physiological NaCl concentrations, resulting in broad-spectrum tolerance to antibiotics.
Figures



Similar articles
-
Phenylacetic acid catabolism modulates virulence factors and drug resistance in Acinetobacter baumannii MCC 2076.World J Microbiol Biotechnol. 2025 Apr 28;41(5):152. doi: 10.1007/s11274-025-04359-x. World J Microbiol Biotechnol. 2025. PMID: 40289051
-
Contribution of EmrAB efflux pumps to colistin resistance in Acinetobacter baumannii.J Microbiol. 2017 Feb;55(2):130-136. doi: 10.1007/s12275-017-6408-5. Epub 2017 Jan 26. J Microbiol. 2017. PMID: 28120193
-
Distribution of different efflux pump genes in clinical isolates of multidrug-resistant Acinetobacter baumannii and their correlation with antimicrobial resistance.J Microbiol Immunol Infect. 2017 Apr;50(2):224-231. doi: 10.1016/j.jmii.2015.04.004. Epub 2015 May 14. J Microbiol Immunol Infect. 2017. PMID: 26055688
-
Multidrug resistant Acinetobacter baumannii--the role of AdeABC (RND family) efflux pump in resistance to antibiotics.Folia Histochem Cytobiol. 2008;46(3):257-67. doi: 10.2478/v10042-008-0056-x. Folia Histochem Cytobiol. 2008. PMID: 19056528 Review.
-
Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art.Expert Rev Anti Infect Ther. 2013 Jun;11(6):571-83. doi: 10.1586/eri.13.38. Expert Rev Anti Infect Ther. 2013. PMID: 23750729 Review.
Cited by
-
Proteomic Analyses of Acinetobacter baumannii Clinical Isolates to Identify Drug Resistant Mechanism.Front Cell Infect Microbiol. 2021 Feb 24;11:625430. doi: 10.3389/fcimb.2021.625430. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33718272 Free PMC article.
-
Acinetobacter spp. porin Omp33-36: Classification and transcriptional response to carbapenems and host cells.PLoS One. 2018 Aug 2;13(8):e0201608. doi: 10.1371/journal.pone.0201608. eCollection 2018. PLoS One. 2018. PMID: 30071077 Free PMC article.
-
NaCl Concentration-Dependent Aminoglycoside Resistance of Halomonas socia CKY01 and Identification of Related Genes.J Microbiol Biotechnol. 2021 Feb 28;31(2):250-258. doi: 10.4014/jmb.2009.09017. J Microbiol Biotechnol. 2021. PMID: 33148940 Free PMC article.
-
Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world?Clin Microbiol Rev. 2013 Apr;26(2):185-230. doi: 10.1128/CMR.00059-12. Clin Microbiol Rev. 2013. PMID: 23554414 Free PMC article. Review.
-
Deciphering the iron response in Acinetobacter baumannii: A proteomics approach.J Proteomics. 2011 Jan 1;74(1):44-58. doi: 10.1016/j.jprot.2010.07.010. Epub 2010 Aug 6. J Proteomics. 2011. PMID: 20692388 Free PMC article.
References
-
- Adams, M. D., K. Goglin, N. Molyneaux, K. M. Hujer, H. Lavender, J. J. Jamison, I. J. MacDonald, K. M. Martin, T. Russo, A. A. Campagnari, A. M. Hujer, R. A. Bonomo, and S. R. Gill. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053-8064. - PMC - PubMed
-
- Adams, M. D., G. C. Nickel, S. Bajaksouzian, H. Lavender, A. R. Murthy, M. R. Jacobs, and R. A. Bonomo. 15 June 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. doi:10.1128/AAC.00284-09. - DOI - PMC - PubMed
-
- Bonomo, R. A., and D. Szabo. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 43(Suppl. 2):S49-S56. - PubMed
-
- Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

