Exploiting the drug-activating properties of a novel trypanosomal nitroreductase
- PMID: 20028822
- PMCID: PMC2825977
- DOI: 10.1128/AAC.01213-09
Exploiting the drug-activating properties of a novel trypanosomal nitroreductase
Abstract
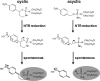
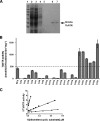
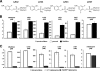
Nitroheterocyclic prodrugs have been used to treat trypanosomal diseases for more than 40 years. Recently, the key step involved in the activation of these compounds has been elucidated and shown to be catalyzed by a type I nitroreductase (NTR). This class of enzyme is normally associated with bacteria and is absent from most eukaryotes, with trypanosomes being a major exception. Here we exploit this difference by evaluating the trypanocidal activity of a library of nitrobenzylphosphoramide mustards against bloodstream-form Trypanosoma brucei parasites. Biochemical screening against the purified enzyme revealed that a subset of halogenated nitroaromatic compounds were effective substrates for T. brucei NTR (TbNTR), having apparent K(cat)/K(m) values approximately 100 times greater than nifurtimox. When tested against T. brucei, cytotoxicity mirrored enzyme activity, with 50% inhibitory concentrations of the most potent substrates being less than 10 nM. T. brucei NTR plays a key role in parasite killing: heterozygous lines displayed resistance to the compounds, while parasites overexpressing the enzyme showed hypersensitivity. We also evaluated the cytotoxicities of substrates with the highest trypanocidal activities by using mammalian THP-1 cells. The relative toxicities of these newly identified compounds were much lower than that of nifurtimox. We conclude that halogenated nitrobenzylphosphoramide mustards represent a novel class of antitrypanosomal agents, and their efficacy validates the strategy of specifically targeting NTR activity to develop new therapeutics.
Figures
References
-
- Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. - PubMed
-
- Arya, S. C., and N. Agarwal. 2009. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 103:994-995. - PubMed
-
- Baliani, A., G. J. Bueno, M. L. Stewart, V. Yardley, R. Brun, M. P. Barrett, and I. H. Gilbert. 2005. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J. Med. Chem. 48:5570-5579. - PubMed
-
- Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

