Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models
- PMID: 20028824
- PMCID: PMC2826009
- DOI: 10.1128/AAC.01114-09
Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models
Abstract
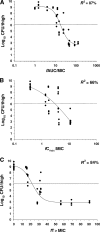
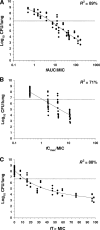
Colistin is increasingly used as last-line therapy against Gram-negative pathogens. The pharmacokinetic (PK)/pharmacodynamic (PD) index that best correlates with the efficacy of colistin remains undefined. The activity of colistin against three strains of Pseudomonas aeruginosa was studied in neutropenic mouse thigh and lung infection models. The PKs of unbound colistin were determined from single-dose PK studies together with extensive plasma protein binding analyses. Dose-fractionation studies were conducted over 24 h with a dose range of 5 to 160 mg/kg of body weight/day. The bacterial burden in the thigh or lung was measured at 24 h after the initiation of treatment. Relationships between antibacterial effect and measures of exposure to unbound (f) colistin (area under the concentration-time curve [fAUC/MIC], maximum concentration of drug in plasma [fC(max)]/MIC, and the time that the concentration in plasma is greater than the MIC [fT > MIC]) were examined by using an inhibitory sigmoid maximum-effect model. Nonlinearity in the PKs of colistin, including its plasma protein binding, was observed. The PK/PD index that correlated best with its efficacy was fAUC/MIC in both the thigh infection model (R(2) = 87%) and the lung infection model (R(2) = 89%). The fAUC/MIC targets required to achieve 1-log and 2-log kill against the three strains were 15.6 to 22.8 and 27.6 to 36.1, respectively, in the thigh infection model, while the corresponding values were 12.2 to 16.7 and 36.9 to 45.9 in the lung infection model. The findings of this in vivo study indicate the importance of achieving adequate time-averaged exposure to colistin. The results will facilitate efforts to define the more rational design of dosage regimens for humans.
Figures

References
-
- Bergen, P. J., J. Li, R. L. Nation, J. D. Turnidge, K. Coulthard, and R. W. Milne. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 61:636-642. - PubMed
-
- Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. - PubMed
-
- Bradley, J. S., R. Guidos, S. Baragona, J. G. Bartlett, E. Rubinstein, G. G. Zhanel, M. D. Tino, D. L. Pompliano, F. Tally, and P. Tipirneni. 2007. Anti-infective research and development—problems, challenges, and solutions. Lancet Infect. Dis. 7:68-78. - PubMed
-
- Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement (M100-S17). Clinical and Laboratory Standards Institute, Wayne, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

