Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals
- PMID: 20028825
- PMCID: PMC2825978
- DOI: 10.1128/AAC.01305-09
Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals
Abstract
Peroxidic antimalarials such as the semisynthetic artemisinins are critically important in the treatment of drug-resistant malaria. Nevertheless, their peroxide bond-dependent mode of action is still not well understood. Using combination experiments with cultured Plasmodium falciparum cells, we investigated the interactions of the nitroxide radical spin trap, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), and four of its analogs with artemisinin and the ozonide drug development candidate OZ277. The antagonism observed for combinations of artemisinin or OZ277 with the TEMPO analogs supports the hypothesis that the formation of carbon-centered radicals is critical for the activity of these two antimalarial peroxides. The TEMPO analogs showed a trend toward greater antagonism with artemisinin than they did with OZ277, an observation that can be explained by the greater tendency of artemisinin-derived carbon-centered radicals to undergo internal self-quenching reactions, resulting in a lower proportion of radicals available for subsequent chemical reactions such as the alkylation of heme and parasite proteins. In a further mechanistic experiment, we tested both artemisinin and OZ277 in combination with their nonperoxidic analogs. The latter had no effect on the antimalarial activities of the former. These data indicate that the antimalarial properties of peroxides do not derive from reversible interactions with parasite targets.
Figures


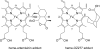
References
-
- Ables, R. H., and A. L. Maycock. 1976. Suicide enzyme inactivators. Acc. Chem. Res. 9:313-319.
-
- Bhisutthibhan, J., X.-Q. Pan, P. A. Hossler, D. J. Walker, C. A. Yowell, J. Carlton, J. R. Dame, and S. R. Meshnick. 1998. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 273:16192-16198. - PubMed
-
- Brossi, A., B. Venugopalan, L. Dominguez Gerpe, H. J. C. Yeh, J. L. Flippen-Anderson, P. Buchs, X. D. Luo, W. K. Milhous, and W. Peters. 1988. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J. Med. Chem. 31:645-650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

