Heat shock protein cognate 70-4 and an E3 ubiquitin ligase, CHIP, mediate plastid-destined precursor degradation through the ubiquitin-26S proteasome system in Arabidopsis
- PMID: 20028838
- PMCID: PMC2814507
- DOI: 10.1105/tpc.109.071548
Heat shock protein cognate 70-4 and an E3 ubiquitin ligase, CHIP, mediate plastid-destined precursor degradation through the ubiquitin-26S proteasome system in Arabidopsis
Abstract
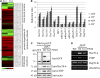
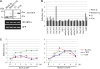
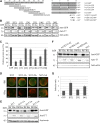
Plastid-targeted proteins pass through the cytosol as unfolded precursors. If proteins accumulate in the cytosol, they can form nonspecific aggregates that cause severe cellular damage. Here, we demonstrate that high levels of plastid precursors are degraded through the ubiquitin-proteasome system (UPS) in Arabidopsis thaliana cells. The cytosolic heat shock protein cognate 70-4 (Hsc70-4) and E3 ligase carboxy terminus of Hsc70-interacting protein (CHIP) were highly induced in plastid protein import2 plants, which had a T-DNA insertion at Toc159 and showed an albino phenotype and a severe defect in protein import into chloroplasts. Hsc70-4 and CHIP together mediated plastid precursor degradation when import-defective chloroplast-targeted reporter proteins were transiently expressed in protoplasts. Hsc70-4 recognized specific sequence motifs in transit peptides and thereby led to precursor degradation through the UPS. CHIP, which interacted with Hsc70-4, functioned as an E3 ligase in the Hsc70-4-mediated protein degradation. The physiological role of Hsc70-4 was confirmed by analyzing Hsc70-4 RNA interference plants in an hsc70-1 mutant background. Plants with lower Hsc70 levels exhibited abnormal embryogenesis, resulting in defective seedlings that displayed high levels of reactive oxygen species and monoubiquitinated Lhcb4 precursors. We propose that Hsc70-4 and CHIP mediate plastid-destined precursor degradation to prevent cytosolic precursor accumulation and thereby play a critical role in embryogenesis.
Figures


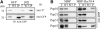

References
-
- Agne, B., and Kessler, F. (2009). Protein transport in organelles: The Toc complex way of preprotein import. FEBS J. 276 1156–1165. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Apel, K., and Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. - PubMed
-
- Baldwin, D., Crane, V., and Rice, D. (1999). A comparison of gel-based, nylon filter and microarray techniques to detect differential RNA expression in plants. Curr. Opin. Plant Biol. 2 96–103. - PubMed
-
- Ballinger, C.A., Connell, P., Wu, Y., Hu, Z., Thompson, L.J., Yin, L.Y., and Patterson, C. (1999). Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 19 4535–4545. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

