Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis
- PMID: 20028840
- PMCID: PMC2814517
- DOI: 10.1105/tpc.109.069435
Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis
Abstract
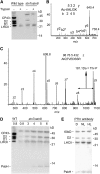
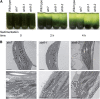
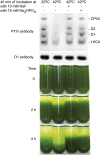
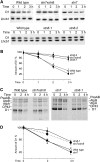
Photosynthetic thylakoid membranes in plants contain highly folded membrane layers enriched in photosystem II, which uses light energy to oxidize water and produce oxygen. The sunlight also causes quantitative phosphorylation of major photosystem II proteins. Analysis of the Arabidopsis thaliana stn7xstn8 double mutant deficient in thylakoid protein kinases STN7 and STN8 revealed light-independent phosphorylation of PsbH protein and greatly reduced N-terminal phosphorylation of D2 protein. The stn7xstn8 and stn8 mutants deficient in light-induced phosphorylation of photosystem II had increased thylakoid membrane folding compared with wild-type and stn7 plants. Significant enhancement in the size of stacked thylakoid membranes in stn7xstn8 and stn8 accelerated gravity-driven sedimentation of isolated thylakoids and was observed directly in plant leaves by transmission electron microscopy. Increased membrane folding, caused by the loss of light-induced protein phosphorylation, obstructed lateral migration of the photosystem II reaction center protein D1 and of processing protease FtsH between the stacked and unstacked membrane domains, suppressing turnover of damaged D1 in the leaves exposed to high light. These findings show that the high level of photosystem II phosphorylation in plants is required for adjustment of macroscopic folding of large photosynthetic membranes modulating lateral mobility of membrane proteins and sustained photosynthetic activity.
Figures
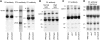


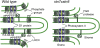
References
-
- Adam, Z., Rudella, A., and van Wijk, K.J. (2006). Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 9 234–240. - PubMed
-
- Albertsson, P.A., and Andreasson, E. (2004). The constant proportion of grana and stroma lamellae in plant chloroplasts. Physiol. Plant. 121 334–342. - PubMed
-
- Andersson, B., and Anderson, J.M. (1980). Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593 427–440. - PubMed
-
- Arntzen, C.J., and Ditto, C.L. (1976). Effects of cations upon chloroplast membrane subunit interactions and excitation energy distribution. Biochim. Biophys. Acta 449 259–274. - PubMed
-
- Aro, E.M., Rokka, A., and Vener, A.V. (2004). Determination of phosphoproteins in higher plant thylakoids. Methods Mol. Biol. 274 271–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

