Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance
- PMID: 20028841
- PMCID: PMC2814500
- DOI: 10.1105/tpc.109.069211
Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance
Abstract
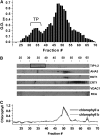
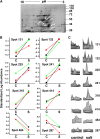
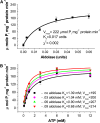
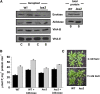
To examine the role of the tonoplast in plant salt tolerance and identify proteins involved in the regulation of transporters for vacuolar Na(+) sequestration, we exploited a targeted quantitative proteomics approach. Two-dimensional differential in-gel electrophoresis analysis of free flow zonal electrophoresis separated tonoplast fractions from control, and salt-treated Mesembryanthemum crystallinum plants revealed the membrane association of glycolytic enzymes aldolase and enolase, along with subunits of the vacuolar H(+)-ATPase V-ATPase. Protein blot analysis confirmed coordinated salt regulation of these proteins, and chaotrope treatment indicated a strong tonoplast association. Reciprocal coimmunoprecipitation studies revealed that the glycolytic enzymes interacted with the V-ATPase subunit B VHA-B, and aldolase was shown to stimulate V-ATPase activity in vitro by increasing the affinity for ATP. To investigate a physiological role for this association, the Arabidopsis thaliana cytoplasmic enolase mutant, los2, was characterized. These plants were salt sensitive, and there was a specific reduction in enolase abundance in the tonoplast from salt-treated plants. Moreover, tonoplast isolated from mutant plants showed an impaired ability for aldolase stimulation of V-ATPase hydrolytic activity. The association of glycolytic proteins with the tonoplast may not only channel ATP to the V-ATPase, but also directly upregulate H(+)-pump activity.
Figures



References
-
- Alban, A., David, S.O., Bjorkesten, L., and Andersson, C. (2003). A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3 36–44. - PubMed
-
- Ames, B.N. (1966). Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8 115–118.
-
- Apse, M.P., Sottosanto, J.B., and Blumwald, E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36 229–239. - PubMed
-
- Barkla, B.J., Vera-Estrella, R., and Pantoja, O. (2007). Enhanced separation of membranes during free flow zonal electrophoresis in plants. Anal. Chem. 79 5181–5187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

