CCL2 blockade augments cancer immunotherapy
- PMID: 20028856
- PMCID: PMC2821565
- DOI: 10.1158/0008-5472.CAN-09-2326
CCL2 blockade augments cancer immunotherapy
Erratum in
- Cancer Res. 2010 Mar 15;70(6):2569. Crisanti, Cecilia [corrected to Crisanti, M Cecilia]
Abstract
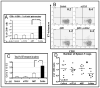
Altering the immunosuppressive microenvironment that exists within a tumor will likely be necessary for cancer vaccines to trigger an effective antitumor response. Monocyte chemoattractant proteins (such as CCL2) are produced by many tumors and have both direct and indirect immunoinhibitory effects. We hypothesized that CCL2 blockade would reduce immunosuppression and augment vaccine immunotherapy. Anti-murine CCL2/CCL12 monoclonal antibodies were administered in three immunotherapy models: one aimed at the human papillomavirus E7 antigen expressed by a non-small cell lung cancer (NSCLC) line, one targeted to mesothelin expressed by a mesothelioma cell line, and one using an adenovirus-expressing IFN-alpha to treat a nonimmunogenic NSCLC line. We evaluated the effect of the combination treatment on tumor growth and assessed the mechanism of these changes by evaluating cytotoxic T cells, immunosuppressive cells, and the tumor microenvironment. Administration of anti-CCL2/CCL12 antibodies along with the vaccines markedly augmented efficacy with enhanced reduction in tumor volume and cures of approximately half of the tumors. The combined treatment generated more total intratumoral CD8+ T cells that were more activated and more antitumor antigen-specific, as measured by tetramer evaluation. Another important potential mechanism was reduction in intratumoral T regulatory cells. CCL2 seems to be a key proximal cytokine mediating immunosuppression in tumors. Its blockade augments CD8+ T-cell immune response to tumors elicited by vaccines via multifactorial mechanisms. These observations suggest that combining CCL2 neutralization with vaccines should be considered in future immunotherapy trials.
Conflict of interest statement
Figures



References
-
- Pardoll D. Does the immune system see tumor as foreign or self? Annual Review of Immunology. 2003;21:807. - PubMed
-
- Haas AR, Sun J, Vachani A, et al. Cycloxygenase-2 Inhibition Augments the Efficacy of a Cancer Vaccine. Clin Cancer Res. 2006;12:214–22. - PubMed
-
- Comes A, Rosso O, Orengo AM, et al. CD25+ Regulatory T Cell Depletion Augments Immunotherapy of Micrometastases by an IL-21-Secreting Cellular Vaccine. J Immunol. 2006;176:1750–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

