Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68
- PMID: 20028857
- PMCID: PMC2884274
- DOI: 10.1158/0008-5472.CAN-09-2788
Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68
Abstract
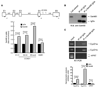
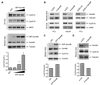
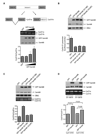
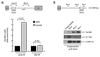
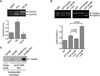
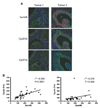
Human cyclin D1 is expressed as two isoforms derived by alternate RNA splicing, termed D1a and D1b, which differ for the inclusion of intron 4 in the D1b mRNA. Both isoforms are frequently upregulated in human cancers, but cyclin D1b displays relatively higher oncogenic potential. The splicing factors that regulate alternative splicing of cyclin D1b remain unknown despite the likelihood that they contribute to cyclin D1 oncogenicity. In this study, we report that Sam68, an RNA-binding protein frequently overexpressed in prostate cancer cells, enhances splicing of cyclin D1b and supports its expression in prostate cancer cells. Chromatin immunoprecipitation and RNA coimmunoprecipitation experiments showed that Sam68 is recruited to the human CCND1 gene encoding cyclin D1 and that it binds to cyclin D1 mRNA. Transient overexpression and RNAi knockdown experiments indicated that Sam68 acts to enhance endogenous expression of cyclin D1b. Minigene reporter assays showed that Sam68 directly affected alternative splicing of CCND1 message, with a preference for the A870 allele that is known to favor cyclin D1b splicing. Sam68 interacted with the proximal region of intron 4, and its binding correlated inversely with recruitment of the spliceosomal component U1-70K. Sam68-mediated splicing was modulated by signal transduction pathways that elicit phosphorylation of Sam68 and regulate its affinity for CCND1 intron 4. Notably, Sam68 expression positively correlates with levels of cyclin D1b, but not D1a, in human prostate carcinomas. Our results identify Sam68 as the first splicing factor to affect CCND1 alternative splicing in prostate cancer cells, and suggest that increased levels of Sam68 may stimulate cyclin D1b expression in human prostate cancers.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
References
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. - PubMed
-
- Li HR, Wang-Rodriguez J, Nair TM, et al. Two-dimensional transcriptome profiling: identification of messenger RNA isoform signatures in prostate cancer from archived paraffin-embedded cancer specimens. Cancer Res. 2006;66:4079–4088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

