c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82
- PMID: 20028971
- PMCID: PMC2825421
- DOI: 10.1074/jbc.M109.038570
c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82
Abstract
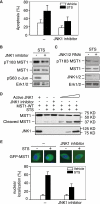
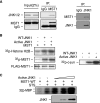
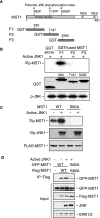
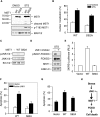
Protein kinases play an important role in the maintenance of homeostasis between cell survival and apoptosis. Deregulation of these kinases leads to various pathological manifestations, such as cancer and neurodegenerative diseases. The MST1 encodes a serine/threonine kinase that is activated upon apoptotic stimulation, which in turn phosphorylates its downstream targets, Histone H2B and FOXO. However, the upstream regulators of MST1 kinase have been poorly studied. In this study, we report that JNK (c-Jun N-terminal kinase) phosphorylates MST1 at serine 82, which leads to the enhancement of MST1 activation. Accordingly, the activation of MST1 phosphorylates FOXO3 at serine 207 and promotes cell death. The inhibition of JNK kinase per se attenuates MST1 activity and nuclear translocation as well as MST1-induced apoptosis. We also find the S82A (serine mutated to alanine) diminishes MST1 activation and its effect on the FOXO transcription activity. Collectively, these findings define the novel feedback regulation of MST1 kinase activation by its putative substrate, JNK, with implication for our understanding of the signaling mechanism during cell death.
Figures
References
-
- Creasy C. L., Ambrose D. M., Chernoff J. (1996) J. Biol. Chem. 271, 21049–21053 - PubMed
-
- Harvey K. F., Pfleger C. M., Hariharan I. K. (2003) Cell 114, 457–467 - PubMed
-
- Wu S., Huang J., Dong J., Pan D. (2003) Cell 114, 445–456 - PubMed
-
- Huang J., Wu S., Barrera J., Matthews K., Pan D. (2005) Cell 122, 421–434 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

