STAT5 activation is critical for the transformation mediated by myeloproliferative disorder-associated JAK2 V617F mutant
- PMID: 20028972
- PMCID: PMC2820758
- DOI: 10.1074/jbc.M109.040733
STAT5 activation is critical for the transformation mediated by myeloproliferative disorder-associated JAK2 V617F mutant
Abstract
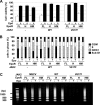
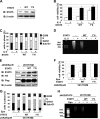
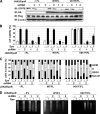
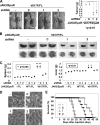
It has been well established that disruption of JAK2 signaling regulation is involved in various hematopoietic disorders; however, the detailed mechanism by which abnormal activation of JAK2 exhibits transforming activity remains to be elucidated. Here, to clarify the functional role of the erythropoietin receptor (EpoR) and its downstream transcription factor STAT5 in the abnormal activation of JAK2-induced hematopoietic diseases, we generated a stable transfectant of Ba/F3 cells expressing EpoR and analyzed the molecular mechanism of how JAK2 mutation induces cell growth disorder. JAK2 V617F mutant exhibited transforming activity when EpoR was coexpressed. According to a study utilizing several truncated mutants of EpoR, the ability of EpoR to facilitate the transforming activity of JAK2 V617F mutant required the intracellular domain to interact with STAT5. Strikingly, once the truncated EpoR (EpoR-H) was mutated on Tyr-343, the phosphorylation of which is known to be important for interaction with STAT5, JAK2 V617F mutant failed to exhibit transforming activity, suggesting that STAT5 is critical for JAK2 mutant-induced hematopoietic disorder. Furthermore, the expression of the constitutively active STAT5 mutant exhibited transforming activity in Ba/F3 cells, and short hairpin RNA-mediated knockdown of STAT5 significantly inhibited the transforming activity of JAK2 V617F mutant. Taking these observations together, STAT5 plays an essential role in EpoR-JAK2 V617F mutant-induced hematopoietic disorder. Although it remains unclear why the presence of EpoR is required to activate oncogenic signaling via the JAK2 mutant and STAT5, its interacting ability is a target for the treatment of these hematopoietic diseases.
Figures





Similar articles
-
Three Tyrosine Residues in the Erythropoietin Receptor Are Essential for Janus Kinase 2 V617F Mutant-induced Tumorigenesis.J Biol Chem. 2017 Feb 3;292(5):1826-1846. doi: 10.1074/jbc.M116.749465. Epub 2016 Dec 20. J Biol Chem. 2017. PMID: 27998978 Free PMC article.
-
Akt activation through the phosphorylation of erythropoietin receptor at tyrosine 479 is required for myeloproliferative disorder-associated JAK2 V617F mutant-induced cellular transformation.Cell Signal. 2011 May;23(5):849-56. doi: 10.1016/j.cellsig.2011.01.009. Epub 2011 Jan 19. Cell Signal. 2011. PMID: 21255641
-
[Analysis of the mechanism of polycythemia vera by studying JAK2 mutant-induced signaling pathway].Yakugaku Zasshi. 2011;131(8):1183-7. doi: 10.1248/yakushi.131.1183. Yakugaku Zasshi. 2011. PMID: 21804321 Review. Japanese.
-
Tyrosine-phosphorylated SOCS3 negatively regulates cellular transformation mediated by the myeloproliferative neoplasm-associated JAK2 V617F mutant.Cytokine. 2019 Nov;123:154753. doi: 10.1016/j.cyto.2019.154753. Epub 2019 Jun 27. Cytokine. 2019. PMID: 31255914
-
JAK2, the JAK2 V617F mutant and cytokine receptors.Pathol Biol (Paris). 2007 Mar;55(2):88-91. doi: 10.1016/j.patbio.2006.06.003. Epub 2006 Aug 14. Pathol Biol (Paris). 2007. PMID: 16904848 Review.
Cited by
-
Malignant JAK-signaling: at the interface of inflammation and malignant transformation.Leukemia. 2025 May;39(5):1011-1030. doi: 10.1038/s41375-025-02569-8. Epub 2025 Mar 26. Leukemia. 2025. PMID: 40140631 Free PMC article. Review.
-
Activation of JAK2-V617F by components of heterodimeric cytokine receptors.J Biol Chem. 2010 May 28;285(22):16651-63. doi: 10.1074/jbc.M109.071191. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363735 Free PMC article.
-
Critical roles of Myc-ODC axis in the cellular transformation induced by myeloproliferative neoplasm-associated JAK2 V617F mutant.PLoS One. 2013;8(1):e52844. doi: 10.1371/journal.pone.0052844. Epub 2013 Jan 3. PLoS One. 2013. PMID: 23300995 Free PMC article.
-
FL118 Is a Potent Therapeutic Agent against Chronic Myeloid Leukemia Resistant to BCR-ABL Inhibitors through Targeting RNA Helicase DDX5.Int J Mol Sci. 2024 Mar 26;25(7):3693. doi: 10.3390/ijms25073693. Int J Mol Sci. 2024. PMID: 38612503 Free PMC article.
-
STAT signaling in the pathogenesis and treatment of myeloid malignancies.JAKSTAT. 2012 Apr 1;1(2):55-64. doi: 10.4161/jkst.20006. JAKSTAT. 2012. PMID: 24058751 Free PMC article. Review.
References
-
- Ihle J. N., Gilliland D. G. (2007) Curr. Opin. Genet. Dev. 17, 8–14 - PubMed
-
- Parganas E., Wang D., Stravopodis D., Topham D. J., Marine J. C., Teglund S., Vanin E. F., Bodner S., Colamonici O. R., van Deursen J. M., Grosveld G., Ihle J. N. (1998) Cell 93, 385–395 - PubMed
-
- Neubauer H., Cumano A., Müller M., Wu H., Huffstadt U., Pfeffer K. (1998) Cell 93, 397–409 - PubMed
-
- Um M., Lodish H. F. (2006) J. Biol. Chem. 281, 5648–5656 - PubMed
-
- Minoo P., Zadeh M. M., Rottapel R., Lebrun J. J., Ali S. (2004) Blood 103, 1398–1407 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

