Ran overexpression leads to diminished T cell responses and selectively modulates nuclear levels of c-Jun and c-Fos
- PMID: 20028981
- PMCID: PMC2820777
- DOI: 10.1074/jbc.M109.058024
Ran overexpression leads to diminished T cell responses and selectively modulates nuclear levels of c-Jun and c-Fos
Abstract
Ras-related nuclear protein (Ran) is a Ras family GTPase, and its documented functions are the regulation of DNA replication, cell cycle progression, nuclear structure formation, RNA processing and exportation, and nuclear protein importation. In this study, we performed detailed mapping of Ran expression during mouse ontogeny using in situ hybridization. High Ran expression was found in various organs and tissues including the thymus cortex and spleen white pulp. Ran was induced in T cells 24 h after their activation. The function of Ran in the immune system was investigated using Ran transgenic (Tg) mice. In Ran Tg T cells, there was compromised activation marker expression, lymphokine secretion, and proliferation upon T cell receptor activation in vitro when compared with wild type T cells. Tg mice also manifested defective delayed type hypersensitivity in vivo. Upon PMA and ionomycin stimulation, Tg T cells were defective in nuclear accumulation of AP-1 factors (c-Jun and c-Fos) but not NF-kappaB family members. Our experiments showed that Ran had important regulatory function in T cell activation. One of the possible mechanisms is that intracellular Ran protein levels control the nuclear retention for selective transcription factors such as c-Jun and c-Fos of AP-1, which is known to be critical in T cell activation and proliferation and lymphokine secretion.
Figures




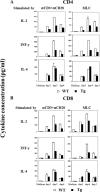

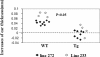

Similar articles
-
Reduced Ran expression in Ran(+/-) fibroblasts increases cytokine-stimulated nuclear abundance of the AP-1 subunits c-Fos and c-Jun.FEBS Lett. 2010 Nov 19;584(22):4623-6. doi: 10.1016/j.febslet.2010.10.030. Epub 2010 Oct 21. FEBS Lett. 2010. PMID: 20965183
-
Impaired induction of c-fos/c-jun genes and of transcriptional regulatory proteins binding distinct c-fos/c-jun promoter elements in activated human T cells during aging.Cell Immunol. 1997 Jan 10;175(1):41-50. doi: 10.1006/cimm.1996.1048. Cell Immunol. 1997. PMID: 9015187
-
Sublethal levels of oxidative stress stimulate transcriptional activation of c-jun and suppress IL-2 promoter activation in Jurkat T cells.J Immunol. 1996 Jul 1;157(1):160-9. J Immunol. 1996. PMID: 8683110
-
MUC1 is a novel costimulatory molecule of human T cells and functions in an AP-1-dependent manner.Hum Immunol. 2012 May;73(5):448-55. doi: 10.1016/j.humimm.2012.02.024. Epub 2012 Mar 6. Hum Immunol. 2012. PMID: 22425740
-
Mechanistic study of saikosaponin-d (Ssd) on suppression of murine T lymphocyte activation.J Cell Biochem. 2009 May 15;107(2):303-15. doi: 10.1002/jcb.22126. J Cell Biochem. 2009. PMID: 19301261
Cited by
-
The Antitumor Effect of Xihuang Pill on Treg Cells Decreased in Tumor Microenvironment of 4T1 Breast Tumor-Bearing Mice by PI3K/AKT~AP-1 Signaling Pathway.Evid Based Complement Alternat Med. 2018 Apr 23;2018:6714829. doi: 10.1155/2018/6714829. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 29849718 Free PMC article.
-
RAN potentiates nuclear export of phosphorylated AMPK, reshaping lipid metabolism and impairing immune efficacy in lung adenocarcinoma.NPJ Precis Oncol. 2025 Jun 6;9(1):165. doi: 10.1038/s41698-025-00977-8. NPJ Precis Oncol. 2025. PMID: 40481101 Free PMC article.
-
Gene Signatures of T-Cell Activation Can Serve as Predictors of Functionality for SARS-CoV-2-Specific T-Cell Receptors.Vaccines (Basel). 2022 Sep 27;10(10):1617. doi: 10.3390/vaccines10101617. Vaccines (Basel). 2022. PMID: 36298482 Free PMC article.
-
Myc and AP-1 expression in T cells and T-cell activation in patients after hematopoietic stem cell transplantation.Clin Exp Med. 2015 May;15(2):189-203. doi: 10.1007/s10238-014-0285-6. Epub 2014 Aug 7. Clin Exp Med. 2015. PMID: 25099175
-
MYCBP2 Is a Guanosine Exchange Factor for Ran Protein and Determines Its Localization in Neurons of Dorsal Root Ganglia.J Biol Chem. 2015 Oct 16;290(42):25620-35. doi: 10.1074/jbc.M115.646901. Epub 2015 Aug 24. J Biol Chem. 2015. PMID: 26304119 Free PMC article.
References
-
- Bischoff F. R., Ponstingl H. (1991) Nature 354, 80–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

