Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury
- PMID: 20029340
- PMCID: PMC2808453
- DOI: 10.1097/CCM.0b013e3181cb1158
Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury
Abstract
Objective: To investigate whether inhibition of cyclooxygenase-2, a critical component of the inflammatory pathway, is neuroprotective in a neonatal rat model of cerebral hypoxia-ischemia. The development of brain inflammation largely contributes to neonatal brain injury that may lead to a lifetime of neurologic deficits.
Design: Laboratory investigation.
Setting: University research laboratory.
Subjects: Postnatal day ten Sprague-Dawley rats.
Interventions: Neonatal hypoxia-ischemia was induced by ligation of the right common carotid artery followed by 2 hrs of hypoxia (8% oxygen). The pups in treatment groups were administered 10 mg/kg (low dose) or 30 mg/kg (high dose) of a known selective cyclooxygenase-2 inhibitor (NS398). Animals were euthanized at three time points: 72 hrs, 2 wks, or 6 wks. Inflammation outcomes were assessed at 72 hrs; brain damage was assessed at 2 wks and 6 wks along with other organs (heart, spleen). Detailed neurobehavioral examination was performed at 6 wks.
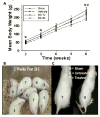
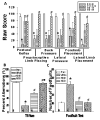
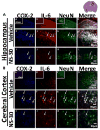
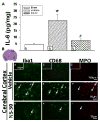
Measurements and main results: Pharmacologic inhibition of cyclooxygenase-2 markedly increased survivability within the first 72 hrs compared with untreated rats (100% vs. 72%). Low- and high-dose NS398 significantly attenuated the loss of brain and body weights observed after hypoxia-ischemia. Neurobehavioral outcomes were significantly improved in some parameters with low-dose treatment, whereas high-dose treatment consistently improved all neurologic deficits. Immunohistochemical results showed a marked decrease in macrophage, microglial, and neutrophil abundance in ipsilateral hemisphere of the NS398-treated group along with a reduction in interleukin-6 expression.
Conclusions: Selective cyclooxygenase-2 inhibition protected neonatal rats against death, progression of brain injury, growth retardation, and neurobehavioral deficits after a hypoxic-ischemic insult.
Conflict of interest statement
Conflict of Interest: None
Figures


Comment in
-
COX2 inhibitors for acquired brain injuries: is the time ripe?Crit Care Med. 2010 Feb;38(2):723-4. doi: 10.1097/CCM.0b013e3181bc80b9. Crit Care Med. 2010. PMID: 20083946 Free PMC article. No abstract available.
Similar articles
-
COX2 inhibitors for acquired brain injuries: is the time ripe?Crit Care Med. 2010 Feb;38(2):723-4. doi: 10.1097/CCM.0b013e3181bc80b9. Crit Care Med. 2010. PMID: 20083946 Free PMC article. No abstract available.
-
Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia.Eur J Pharmacol. 2002 Feb 22;437(3):139-45. doi: 10.1016/s0014-2999(02)01289-x. Eur J Pharmacol. 2002. PMID: 11890901
-
Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat.Eur J Neurosci. 2006 Jul;24(2):341-50. doi: 10.1111/j.1460-9568.2006.04918.x. Epub 2006 Jul 12. Eur J Neurosci. 2006. PMID: 16836639
-
Neuroprotective effect of the peptides ADNF-9 and NAP on hypoxic-ischemic brain injury in neonatal rats.Brain Res. 2006 Oct 18;1115(1):169-78. doi: 10.1016/j.brainres.2006.07.114. Epub 2006 Aug 30. Brain Res. 2006. PMID: 16938277
-
Splenic immune cells in experimental neonatal hypoxia-ischemia.Transl Stroke Res. 2013 Apr;4(2):208-19. doi: 10.1007/s12975-012-0239-9. Transl Stroke Res. 2013. PMID: 23626659 Free PMC article.
Cited by
-
Baby STEPS: a giant leap for cell therapy in neonatal brain injury.Pediatr Res. 2011 Jul;70(1):3-9. doi: 10.1203/PDR.0b013e31821d0d00. Pediatr Res. 2011. PMID: 21659957 Free PMC article. Review.
-
Ameliorative effects of 6‑gingerol in cerebral ischemia are mediated via the activation of antioxidant and anti‑inflammatory pathways.Biomed Rep. 2023 Feb 15;18(4):26. doi: 10.3892/br.2023.1608. eCollection 2023 Apr. Biomed Rep. 2023. PMID: 36909941 Free PMC article.
-
A novel preclinical model of germinal matrix hemorrhage using neonatal rats.Acta Neurochir Suppl. 2011;111:55-60. doi: 10.1007/978-3-7091-0693-8_10. Acta Neurochir Suppl. 2011. PMID: 21725732 Free PMC article.
-
Neuroprotection by melatonin after germinal matrix hemorrhage in neonatal rats.Acta Neurochir Suppl. 2011;111:201-6. doi: 10.1007/978-3-7091-0693-8_34. Acta Neurochir Suppl. 2011. PMID: 21725756 Free PMC article.
-
The immune response after hypoxia-ischemia in a mouse model of preterm brain injury.J Neuroinflammation. 2014 Sep 5;11:153. doi: 10.1186/s12974-014-0153-z. J Neuroinflammation. 2014. PMID: 25187205 Free PMC article.
References
-
- Wagner BP, Nedelcu J, Martin E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res. 2002;51:354–360. - PubMed
-
- Lubics A, Reglodi D, Tamas A, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165. - PubMed
-
- Shaywitz BA, Fletcher JM. Neurological, cognitive, and behavioral sequelae of hypoxic-ischemic encephalopathy. Semin Perinatol. 1993;17:357–366. - PubMed
-
- Calvert JW, Zhang JH. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurol Res. 2005;27:246–260. - PubMed
-
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

