BRCA1 and its toolbox for the maintenance of genome integrity
- PMID: 20029420
- PMCID: PMC3899800
- DOI: 10.1038/nrm2831
BRCA1 and its toolbox for the maintenance of genome integrity
Abstract
The breast and ovarian cancer type 1 susceptibility protein (BRCA1) has pivotal roles in the maintenance of genome stability. Studies support that BRCA1 exerts its tumour suppression function primarily through its involvement in cell cycle checkpoint control and DNA damage repair. In addition, recent proteomic and genetic studies have revealed the presence of distinct BRCA1 complexes in vivo, each of which governs a specific cellular response to DNA damage. Thus, BRCA1 is emerging as the master regulator of the genome through its ability to execute and coordinate various aspects of the DNA damage response.
Figures

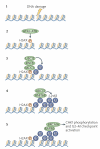
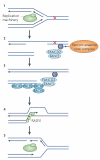
References
-
- Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
-
- Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. - PubMed
-
- Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
-
-
Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. Together with reference 6, these studies laid the groundwork for how the BRCA1 BRCT domains interact with various binding partners.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

