Senescence in tumours: evidence from mice and humans
- PMID: 20029423
- PMCID: PMC3672965
- DOI: 10.1038/nrc2772
Senescence in tumours: evidence from mice and humans
Abstract
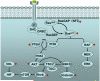
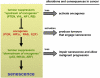
The importance of cellular senescence, which is a stress response that stably blocks proliferation, is increasingly being recognized. Senescence is prevalent in pre-malignant tumours, and progression to malignancy requires evading senescence. Malignant tumours, however, may still undergo senescence owing to interventions that restore tumour suppressor function or inactivate oncogenes. Senescent tumour cells can be cleared by immune cells, which may result in efficient tumour regression. Standard chemotherapy also has the potential to induce senescence, which may partly underlie its therapeutic activity. Although these concepts are well supported in mouse models, translating them to clinical oncology remains a challenge.
Figures
References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. - PubMed
-
-
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. Original description of oncogene-induced senescence in in vitro cultured primary human and mouse cells after overexpression of oncogenic HRAS. Prompted the concept of cellular senescence as a tumour suppressor mechanism.
-
-
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. - PubMed
-
- Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

