Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation
- PMID: 20029461
- PMCID: PMC4003257
- DOI: 10.1038/cmi.2009.106
Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation
Abstract
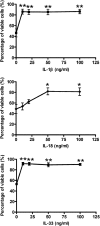
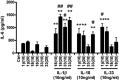
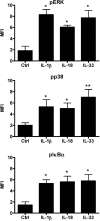
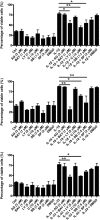
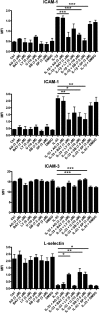
The novel interleukin (IL)-1 family cytokine IL-33 has been shown to activate T helper 2 (Th2) lymphocytes, mast cells and basophils to produce an array of proinflammatory cytokines, as well as to mediate blood eosinophilia, IgE secretion and hypertrophy of airway epithelium in mice. In the present study, we characterized the activation of human eosinophils by IL-33, and investigated the underlying intracellular signaling mechanisms. IL-33 markedly enhanced eosinophil survival and upregulated cell surface expression of the adhesion molecule intercellular adhesion molecule (ICAM)-1 on eosinophils, but it suppressed that of ICAM-3 and L-selectin. In addition, IL-33 mediates significant release of the proinflammatory cytokine IL-6 and the chemokines CXCL8 and CCL2. We found that IL-33-mediated enhancement of survival, induction of adhesion molecules, and release of cytokines and chemokines were differentially regulated by activation of the nuclear factor (NF)-kappaB, p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) pathways. Furthermore, we compared the above IL-33 activities with two structurally and functionally related cytokines, IL-1beta and IL-18. IL-1beta, but not IL-18, markedly upregulated cell surface expression of ICAM-1. IL-1beta and IL-18 also significantly enhanced eosinophil survival, and induced the release of IL-6 and chemokines CXCL8 and CCL2 via the activation of the NF-kappaB, p38 MAPK and ERK pathways. Synergistic effects on the release of IL-6 were also observed in combined treatment with IL-1beta, IL-18 and IL-33. Taken together, our findings provide insight into IL-33-mediated activation of eosinophils via differential intracellular signaling cascades in the immunopathogenesis of allergic inflammation.
Figures



Similar articles
-
Activation of eosinophils by IL-12 family cytokine IL-27: Implications of the pleiotropic roles of IL-27 in allergic responses.Immunobiology. 2011 Jan-Feb;216(1-2):54-65. doi: 10.1016/j.imbio.2010.03.004. Epub 2010 Mar 16. Immunobiology. 2011. PMID: 20435369
-
IL-25 regulates the expression of adhesion molecules on eosinophils: mechanism of eosinophilia in allergic inflammation.Allergy. 2006 Jul;61(7):878-85. doi: 10.1111/j.1398-9995.2006.01102.x. Allergy. 2006. PMID: 16792588
-
Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation.J Immunol. 2008 Apr 15;180(8):5625-35. doi: 10.4049/jimmunol.180.8.5625. J Immunol. 2008. PMID: 18390747
-
The role of human mast cell-derived cytokines in eosinophil biology.J Interferon Cytokine Res. 2004 May;24(5):271-81. doi: 10.1089/107999004323065057. J Interferon Cytokine Res. 2004. PMID: 15153310 Review.
-
Interleukin 25 in allergic airway inflammation.Int Arch Allergy Immunol. 2006;140 Suppl 1:59-62. doi: 10.1159/000092713. Epub 2006 Jun 9. Int Arch Allergy Immunol. 2006. PMID: 16772729 Review.
Cited by
-
Eosinophils and IL-33 Perpetuate Chronic Inflammation and Fibrosis in a Pediatric Population with Stricturing Crohn's Ileitis.Inflamm Bowel Dis. 2015 Oct;21(10):2429-40. doi: 10.1097/MIB.0000000000000512. Inflamm Bowel Dis. 2015. PMID: 26218140 Free PMC article.
-
A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System.Transfus Med Hemother. 2016 Mar;43(2):96-108. doi: 10.1159/000445215. Epub 2016 Mar 18. Transfus Med Hemother. 2016. PMID: 27226792 Free PMC article. Review.
-
Inflammatory cytokine signalling in vulvovaginal candidiasis: a hot mess driving immunopathology.Oxf Open Immunol. 2024 Aug 17;5(1):iqae010. doi: 10.1093/oxfimm/iqae010. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39234208 Free PMC article. Review.
-
Effects of interleukin-33 on cardiac fibroblast gene expression and activity.Cytokine. 2012 Jun;58(3):368-79. doi: 10.1016/j.cyto.2012.02.008. Epub 2012 Mar 23. Cytokine. 2012. PMID: 22445500 Free PMC article.
-
Mechanistic Insights into Eosinophilic Esophagitis: Therapies Targeting Pathophysiological Mechanisms.Cells. 2023 Oct 18;12(20):2473. doi: 10.3390/cells12202473. Cells. 2023. PMID: 37887317 Free PMC article. Review.
References
-
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan T, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
-
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Kondo Y, Yoshimoto T, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. - PubMed
-
- Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia C, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. - PubMed
-
- Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–1881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

