Arginase in parasitic infections: macrophage activation, immunosuppression, and intracellular signals
- PMID: 20029630
- PMCID: PMC2792949
- DOI: 10.1155/2010/683485
Arginase in parasitic infections: macrophage activation, immunosuppression, and intracellular signals
Abstract
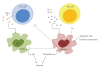
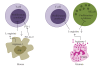
A type 1 cytokine-dependent proinflammatory response inducing classically activated macrophages (CaMvarphis) is crucial for parasite control during protozoan infections but can also contribute to the development of immunopathological disease symptoms. Type 2 cytokines such as IL-4 and IL-13 antagonize CaMvarphis inducing alternatively activated macrophages (AaMvarphis) that upregulate arginase-1 expression. During several infections, induction of arginase-1-macrophages was showed to have a detrimental role by limiting CaMvarphi-dependent parasite clearance and promoting parasite proliferation. Additionally, the role of arginase-1 in T cell suppression has been explored recently. Arginase-1 can also be induced by IL-10 and transforming growth factor-beta (TGF-beta) or even directly by parasites or parasite components. Therefore, generation of alternative activation states of macrophages could limit collateral tissue damage because of excessive type 1 inflammation. However, they affect disease outcome by promoting parasite survival and proliferation. Thus, modulation of macrophage activation may be instrumental in allowing parasite persistence and long-term host survival.
Figures
Similar articles
-
Alternatively activated macrophages in protozoan infections.Curr Opin Immunol. 2007 Aug;19(4):454-9. doi: 10.1016/j.coi.2007.05.007. Epub 2007 Jul 12. Curr Opin Immunol. 2007. PMID: 17628461 Review.
-
[Macrophages and arginase induction as a mechanism for parasite escape].Medicina (B Aires). 2007;67(6 Pt 2):737-46. Medicina (B Aires). 2007. PMID: 18422071 Spanish.
-
Regulatory Macrophages Inhibit Alternative Macrophage Activation and Attenuate Pathology Associated with Fibrosis.J Immunol. 2019 Oct 15;203(8):2130-2140. doi: 10.4049/jimmunol.1900270. Epub 2019 Sep 20. J Immunol. 2019. PMID: 31541024
-
Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation.J Leukoc Biol. 2005 Mar;77(3):321-7. doi: 10.1189/jlb.0304212. Epub 2004 Dec 9. J Leukoc Biol. 2005. PMID: 15591125
-
Alternative activation of macrophages: immune function and cellular biology.Immunobiology. 2009;214(7):630-41. doi: 10.1016/j.imbio.2008.11.009. Epub 2009 Mar 5. Immunobiology. 2009. PMID: 19264378 Review.
Cited by
-
Model-driven multi-omic data analysis elucidates metabolic immunomodulators of macrophage activation.Mol Syst Biol. 2012 Jun 26;8:558. doi: 10.1038/msb.2012.21. Mol Syst Biol. 2012. PMID: 22735334 Free PMC article.
-
Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth.Cancer Res. 2013 Mar 15;73(6):1752-63. doi: 10.1158/0008-5472.CAN-12-2474. Epub 2013 Jan 17. Cancer Res. 2013. PMID: 23328584 Free PMC article.
-
Arginase promotes immune evasion of Echinococcus granulosus in mice.Parasit Vectors. 2020 Feb 6;13(1):49. doi: 10.1186/s13071-020-3919-4. Parasit Vectors. 2020. PMID: 32029006 Free PMC article.
-
Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms.Am J Surg Pathol. 2010 Aug;34(8):1147-54. doi: 10.1097/PAS.0b013e3181e5dffa. Am J Surg Pathol. 2010. PMID: 20661013 Free PMC article.
-
The genetics of Leishmania virulence.Med Microbiol Immunol. 2015 Dec;204(6):619-34. doi: 10.1007/s00430-015-0422-1. Epub 2015 Jun 6. Med Microbiol Immunol. 2015. PMID: 26047933 Review.
References
-
- Mosser DM. The many faces of macrophage activation. Journal of Leukocyte Biology. 2003;73(2):209–212. - PubMed
-
- Nathan C. Mechanisms and modulation of macrophage activation. Behring Institut Mitteilungen. 1991;(88):200–207. - PubMed
-
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259(5102):1739–1742. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3(1):23–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

