Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle
- PMID: 20029633
- PMCID: PMC2793424
- DOI: 10.1155/2009/135249
Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle
Abstract
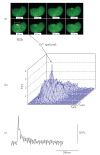
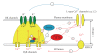
Calcium sparks represent local, rapid, and transient calcium release events from a cluster of ryanodine receptors (RyRs) in the sarcoplasmic reticulum. In arterial smooth muscle cells (SMCs), calcium sparks activate calcium-dependent potassium channels causing decrease in the global intracellular [Ca2+] and oppose vasoconstriction. This is in contrast to cardiac and skeletal muscle, where spatial and temporal summation of calcium sparks leads to global increases in intracellular [Ca2+] and myocyte contraction. We summarize the present data on local RyR calcium signaling in arterial SMCs in comparison to striated muscle and muscle-specific differences in coupling between L-type calcium channels and RyRs. Accordingly, arterial SMC Ca(v)1.2 L-type channels regulate intracellular calcium stores content, which in turn modulates calcium efflux though RyRs. Downregulation of RyR2 up to a certain degree is compensated by increased SR calcium content to normalize calcium sparks. This indirect coupling between Ca(v)1.2 and RyR in arterial SMCs is opposite to striated muscle, where triggering of calcium sparks is controlled by rapid and direct cross-talk between Ca(v)1.1/Ca(v)1.2 L-type channels and RyRs. We discuss the role of RyR isoforms in initiation and formation of calcium sparks in SMCs and their possible molecular binding partners and regulators, which differ compared to striated muscle.
Figures


References
-
- Cheng H, Lederer WJ. Calcium sparks. Physiological Reviews. 2008;88(4):1491–1545. - PubMed
-
- Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. - PubMed
-
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21(1):69–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

