P2X(7) Receptors in Neurological and Cardiovascular Disorders
- PMID: 20029634
- PMCID: PMC2794459
- DOI: 10.1155/2009/861324
P2X(7) Receptors in Neurological and Cardiovascular Disorders
Abstract
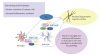
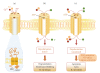
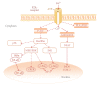
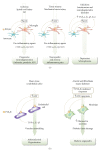
P2X receptors are ATP-gated cation channels that mediate fast excitatory transmission in diverse regions of the brain and spinal cord. Several P2X receptor subtypes, including P2X(7), have the unusual property of changing their ion selectivity during prolonged exposure to ATP, which results in a channel pore permeable to molecules as large as 900 daltons. The P2X(7) receptor was originally described in cells of hematopoietic origin, and mediates the influx of Ca(2+) and Na(+) and Ca(2+) and Na(+) ions as well as the release of proinflammatory cytokines. P2X(7) receptors may affect neuronal cell death through their ability to regulate the processing and release of interleukin-1beta, a key mediator in neurodegeneration, chronic inflammation, and chronic pain. Activation of P2X(7), a key mediator in neurodegeneration, chronic inflammation, and chronic pain. Activation of P2X(7) receptors provides an inflammatory stimulus, and P2X(7) receptor-deficient mice have substantially attenuated inflammatory responses, including models of neuropathic and chronic inflammatory pain. Moreover, P2X(7) receptor activity, by regulating the release of proinflammatory cytokines, may be involved in the pathophysiology of depression. Apoptotic cell death occurs in a number of vascular diseases, including atherosclerosis, restenosis, and hypertension, and may be linked to the release of ATP from endothelial cells, P2X(7) receptor activation, proinflammatory cytokine production, and endothelial cell apoptosis. In this context, the P2X(7) receptor may be viewed as a gateway of communication between the nervous, immune, and cardiovascular systems.
Figures

Similar articles
-
The P2X7 purinergic receptor: from physiology to neurological disorders.FASEB J. 2010 Feb;24(2):337-45. doi: 10.1096/fj.09-138883. Epub 2009 Oct 7. FASEB J. 2010. PMID: 19812374 Review.
-
P2X7 receptors: channels, pores and more.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):705-21. doi: 10.2174/187152712803581137. CNS Neurol Disord Drug Targets. 2012. PMID: 22963440 Review.
-
P2X(7) Receptors as a Transducer in the Co-Occurrence of Neurological/Psychiatric and Cardiovascular Disorders: A Hypothesis.Cardiovasc Psychiatry Neurol. 2009;2009:545263. doi: 10.1155/2009/545263. Epub 2009 Aug 10. Cardiovasc Psychiatry Neurol. 2009. PMID: 20029625 Free PMC article.
-
Proteomics to identify proteins interacting with P2X2 ligand-gated cation channels.J Vis Exp. 2009 May 18;(27):1178. doi: 10.3791/1178. J Vis Exp. 2009. PMID: 19455095 Free PMC article.
-
ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels.J Neurosci. 2002 Apr 15;22(8):3061-9. doi: 10.1523/JNEUROSCI.22-08-03061.2002. J Neurosci. 2002. PMID: 11943809 Free PMC article.
Cited by
-
Loss of function mutation in the P2X7, a ligand-gated ion channel gene associated with hypertrophic cardiomyopathy.Purinergic Signal. 2019 Jun;15(2):205-210. doi: 10.1007/s11302-019-09660-7. Epub 2019 May 31. Purinergic Signal. 2019. PMID: 31152337 Free PMC article.
-
Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications.Neuroscience. 2013 Aug 29;246:199-229. doi: 10.1016/j.neuroscience.2013.04.060. Epub 2013 May 3. Neuroscience. 2013. PMID: 23644052 Free PMC article. Review.
-
Targeting anti-inflammatory treatment can ameliorate injury-induced neuropathic pain.PLoS One. 2013;8(2):e57721. doi: 10.1371/journal.pone.0057721. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469058 Free PMC article.
-
TNF-Α may mediate inflammasome activation in the absence of bacterial infection in more than one way.PLoS One. 2013 Aug 7;8(8):e71477. doi: 10.1371/journal.pone.0071477. Print 2013. PLoS One. 2013. PMID: 23940760 Free PMC article.
-
P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice.J Clin Invest. 2011 Jul;121(7):2932-44. doi: 10.1172/JCI46129. Epub 2011 Jun 13. J Clin Invest. 2011. PMID: 21670495 Free PMC article.
References
-
- Craft JM, Watterson DM, van Eldik LJ. Neuroinflammation: a potential therapeutic target. Expert Opinion on Therapeutic Targets. 2005;9(5):887–900. - PubMed
-
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–432. - PubMed
-
- McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Archives of Neurology. 2001;58(11):1790–1792. - PubMed
-
- Eikelenboom P, van Gool WA. Neuroinflammatory perspectives on the two faces of Alzheimer's disease. Journal of Neural Transmission. 2004;111(3):281–294. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
