Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R alphaA-crystallin
- PMID: 20029648
- PMCID: PMC2796635
Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R alphaA-crystallin
Abstract
Purpose: Crystallins are major functional and structural proteins in mammalian lens. Their expression, distribution, and protein-protein interaction affect lens development and fiber cell differentiation. Mutated crystallins lead to structural and functional changes of lens structure and could lead to opacity formation and cataract development. The purpose of this study was to investigate the biological effects of the cataract-causing G98R mutation on the alphaA-crystallin (CRYAA) protein and to test the capability of chemical chaperone trimethylamine N-oxide (TMAO) to reverse such effects.
Methods: Myc/His-tagged, human, full-length, wild-type (WT) or G98R CRYAA was expressed in human lens epithelial B3 cells and treated or not treated with TMAO. Triton X-100 (Tx) solubility and cellular localization of CRYAA were examined by western blotting and confocal immunofluorescence, respectively. Ubiquitin proteasome-associated degradation was assayed by MG132 treatment. Endoplasmic reticulum (ER) stress, unfolded protein response, and apoptosis were analyzed by the expression of phosphorylated protein kinase-like ER-kinase, binding immunoglobulin protein (BiP), C/EBP homologous protein/growth arrest and DNA damage-inducible gene 153 (CHOP/GADD153), and caspase-3 and immunocytochemistry. Changes in heat shock and stress signaling were investigated.
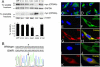
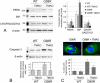
Results: When transfected in lens epithelial B3 cells, unlike WT CRYAA located in the cytoplasm, the G98R CRYAA mutant formed aggregates inside the ER and the protein was predominantly Tx-insoluble. ER stress was induced by G98R CRYAA expression, and cells underwent apoptosis, as shown by a more frequent appearance of fragmented nuclei. Treatment with TMAO reduced Tx-insoluble mutant protein in time- and dose-dependent manners. Other chemical chaperones, 4-phenylbutyric acid, dimethysulfoxide, and glycerol, were much less effective than TMAO. ER-associated aggregates were reduced after TMAO treatment, and the protein was degraded through the ubiquitin-proteasome pathway. This alleviated ER stress and resulted in less apoptosis. Moreover, TMAO treatment induced a moderate upregulation of heat shock protein 70, indicating its effect on heat-shock response to modulate protein folding and assembly. No change was found for nontransfected cells after TMAO treatment.
Conclusion: The natural osmolyte and chemical chaperone TMAO reduced the aggregation of G98R CRYAA. This alleviated ER stress and rescued the affected cells from apoptosis. Our results showed that the chemical chaperone reduces mutant CRYAA aggregates in lens cells. We suggest a potential chemical-based strategy to reduce lens opacity formation.
Figures

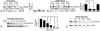


Similar articles
-
Synergistic effects of metal ion and the pre-senile cataract-causing G98R alphaA-crystallin: self-aggregation propensities and chaperone activity.Mol Vis. 2009 Oct 16;15:2050-60. Mol Vis. 2009. PMID: 19862354 Free PMC article.
-
Congenital cataract causing mutants of αA-crystallin/sHSP form aggregates and aggresomes degraded through ubiquitin-proteasome pathway.PLoS One. 2011;6(11):e28085. doi: 10.1371/journal.pone.0028085. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140512 Free PMC article.
-
The cataract-causing mutation G98R in human alphaA-crystallin leads to folding defects and loss of chaperone activity.Mol Vis. 2006 Nov 15;12:1372-9. Mol Vis. 2006. PMID: 17149363
-
Effects of alpha-crystallin on lens cell function and cataract pathology.Curr Mol Med. 2009 Sep;9(7):887-92. doi: 10.2174/156652409789105598. Curr Mol Med. 2009. PMID: 19860667 Review.
-
Genetics of crystallins: cataract and beyond.Exp Eye Res. 2009 Feb;88(2):173-89. doi: 10.1016/j.exer.2008.10.011. Epub 2008 Nov 1. Exp Eye Res. 2009. PMID: 19007775 Review.
Cited by
-
The gut metabolite, trimethylamine N-oxide inhibits protein folding by affecting cis-trans isomerization and induces cell cycle arrest.Cell Mol Life Sci. 2021 Dec 25;79(1):12. doi: 10.1007/s00018-021-04087-z. Cell Mol Life Sci. 2021. PMID: 34953141 Free PMC article.
-
Two novel mutations identified in ADCC families impair crystallin protein distribution and induce apoptosis in human lens epithelial cells.Sci Rep. 2017 Dec 19;7(1):17848. doi: 10.1038/s41598-017-18222-z. Sci Rep. 2017. PMID: 29259299 Free PMC article.
-
Sodium 4-phenylbutyrate ameliorates the effects of cataract-causing mutant gammaD-crystallin in cultured cells.Mol Vis. 2010 Jun 4;16:997-1003. Mol Vis. 2010. PMID: 20577655 Free PMC article.
-
GJA8 missense mutation disrupts hemichannels and induces cell apoptosis in human lens epithelial cells.Sci Rep. 2019 Dec 16;9(1):19157. doi: 10.1038/s41598-019-55549-1. Sci Rep. 2019. PMID: 31844091 Free PMC article.
-
Tang-Luo-Ning, a Traditional Chinese Medicine, Inhibits Endoplasmic Reticulum Stress-Induced Apoptosis of Schwann Cells under High Glucose Environment.Evid Based Complement Alternat Med. 2017;2017:5193548. doi: 10.1155/2017/5193548. Epub 2017 Nov 21. Evid Based Complement Alternat Med. 2017. PMID: 29362588 Free PMC article.
References
-
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. - PubMed
-
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–7. - PubMed
-
- Wang K, Cheng C, Li L, Liu H, Huang Q, Xia CH, Yao K, Sun P, Horwitz J, Gong X. GammaD-crystallin associated protein aggregation and lens fiber cell denucleation. Invest Ophthalmol Vis Sci. 2007;48:3719–28. - PubMed
-
- Andley UP. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. - PubMed
-
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83:508–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
