HIV-1 reverse transcriptase drug-resistance mutations in chronically infected individuals receiving or naïve to HAART in Cameroon
- PMID: 20029816
- PMCID: PMC2958705
- DOI: 10.1002/jmv.21677
HIV-1 reverse transcriptase drug-resistance mutations in chronically infected individuals receiving or naïve to HAART in Cameroon
Abstract
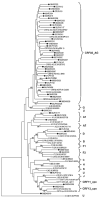
The most common first-line, highly active anti-retroviral therapy (HAART) received by individuals infected with HIV-1 in Cameroon is the combination therapy Triomune, comprised of two nucleoside reverse transcriptase inhibitors (NRTI) and one non-NRTI (NNRTI). To examine the efficacy of these drugs in Cameroon, where diverse non-B HIV-1 subtypes and recombinant viruses predominate, the reverse transcriptase (RT) viral sequences in patient plasma were analyzed for the presence of mutations that confer drug resistance. Forty-nine HIV-1-positive individuals were randomly selected from those receiving care in HIV/AIDS outpatient clinics in the South-West and North-West Regions of Cameroon. Among the 28 patients receiving HAART, 39% (11/28) had resistance to NRTIs, and 46% (13/28) to NNRTIs after a median of 12 months from the start of therapy. Among those with drug-resistance mutations, there was a median of 14 months from the start of HAART, versus 9 months for those without; no difference was observed in the average viral load (10,997 copies/ml vs. 8,056 copies/ml). In contrast, drug-naïve individuals had a significantly higher average viral load (27,929 copies/ml) than those receiving HAART (9,527 copies/ml). Strikingly, among the 21 drug-naïve individuals, 24% harbored viruses with drug-resistance mutations, suggesting that HIV-1 drug-resistant variants are being transmitted in Cameroon. Given the high frequency of resistance mutations among those on first-line HAART, coupled with the high prevalence of HIV-1 variants with drug-resistance mutations among drug-naïve individuals, this study emphasizes the need for extensive monitoring of resistance mutations and the introduction of a second-line HAART strategy in Cameroon.
(c) 2009 Wiley-Liss, Inc.
Figures
References
-
- Adje C, Cheingsong R, Roels TH, Maurice C, Djomand G, Verbiest W, Hertogs K, Larder B, Monga B, Peeters M, Eholie S, Bissagene E, Coulibaly M, Respess R, Wiktor SZ, Chorba T, Nkengasong JN. High prevalence of genotypic and phenotypic HIV-1 drug-resistant strains among patients receiving antiretroviral therapy in Abidjan, Cote d'Ivoire. J Acquir Immune Defic Syndr. 2001;26:501–506. - PubMed
-
- Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: Multicentric observational cohort. AIDS. 2006;20:1163–1169. - PubMed
-
- Delaugerre C, Peytavin G, Dominguez S, Marcelin AG, Duvivier C, Gourlain K, Amellal B, Legrand M, Raffi F, Costagliola D, Katlama C, Calvez V. Virological and pharmacological factors associated with virological response to salvage therapy after an 8-week of treatment interruption in a context of very advanced HIV disease (GigHAART ANRS 097) J Med Virol. 2005;77:345–350. - PubMed
-
- Derache A, Maiga AI, Traore O, Akonde A, Cisse M, Jarrousse B, Koita V, Diarra B, Carcelain G, Barin F, Pizzocolo C, Pizarro L, Katlama C, Calvez V, Marcelin AG. Evolution of genetic diversity and drug resistance mutations in HIV-1 among untreated patients from Mali between 2005 and 2006. J Antimicrob Chemother. 2008;62:456–463. - PubMed
-
- Gallant JE. Drug resistance after failure of initial antiretroviral therapy in resource-limited countries. Clin Infect Dis. 2007;44:453–455. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

