An improved SUMmOn-based methodology for the identification of ubiquitin and ubiquitin-like protein conjugation sites identifies novel ubiquitin-like protein chain linkages
- PMID: 20029837
- PMCID: PMC3374337
- DOI: 10.1002/pmic.200900648
An improved SUMmOn-based methodology for the identification of ubiquitin and ubiquitin-like protein conjugation sites identifies novel ubiquitin-like protein chain linkages
Abstract
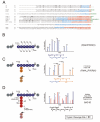
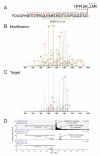
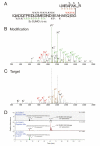
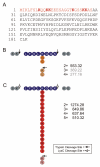
Ubiquitin (Ub) and the ubiquitin-like proteins (Ubls) comprise a remarkable assortment of polypeptides that are covalently conjugated to target proteins (or other biomolecules) to modulate their intracellular localization, half-life, and/or activity. Identification of Ub/Ubl conjugation sites on a protein of interest can thus be extremely important for understanding how it is regulated. While MS has become a powerful tool for the study of many classes of PTMs, the identification of Ub/Ubl conjugation sites presents a number of unique challenges. Here, we present an improved Ub/Ubl conjugation site identification strategy, utilizing SUMmOn analysis and an additional protease (lysyl endopeptidase C), as a complement to standard approaches. As compared with standard trypsin proteolysis-database search protocols alone, the addition of SUMmOn analysis can (i) identify Ubl conjugation sites that are not detected by standard database searching methods, (ii) better preserve Ub/Ubl conjugate identity, and (iii) increase the number of identifications of Ub/Ubl modifications in lysine-rich protein regions. Using this methodology, we characterize for the first time a number of novel Ubl linkages and conjugation sites, including alternative yeast (K54) and mammalian small ubiquitin-related modifier (SUMO) chain (SUMO-2 K42, SUMO-3 K41) assemblies, as well as previously unreported NEDD8 chain (K27, K33, and K54) topologies.
Figures

References
-
- Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003;28:321–328. - PubMed
-
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. - PubMed
-
- Ciechanover A, Iwai K. The ubiquitin system: from basic mechanisms to the patient bed. IUBMB Life. 2004;56:193–201. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

