Carbonyl imines from oxaziridines: generation and cycloaddition of N-O=C dipoles
- PMID: 20029866
- PMCID: PMC3057062
- DOI: 10.1002/anie.200905801
Carbonyl imines from oxaziridines: generation and cycloaddition of N-O=C dipoles
Figures
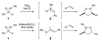
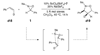
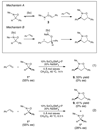
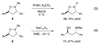
Similar articles
-
NaI-catalyzed highly regioselective ring-opening [1 + 2] cycloaddition reaction of cyclopropenes with imines: highly stereoselective synthesis of cis-vinylic aziridines.Chem Commun (Camb). 2005 Feb 21;(7):909-11. doi: 10.1039/b413807d. Epub 2005 Jan 5. Chem Commun (Camb). 2005. PMID: 15700078
-
Scandium triflate catalyzed cycloaddition of imines with 1,1-cyclopropanediesters: efficient and diastereoselective synthesis of multi-substituted pyrrolidines.Org Biomol Chem. 2006 Jan 21;4(2):299-301. doi: 10.1039/b512195g. Epub 2005 Dec 6. Org Biomol Chem. 2006. PMID: 16391772
-
An efficient, overall [4+1] cycloaddition of 1,3-dienes and nitrene precursors.Chemistry. 2011 Oct 4;17(41):11553-8. doi: 10.1002/chem.201101630. Epub 2011 Sep 2. Chemistry. 2011. PMID: 21887836
-
3-Arylaziridine-2-carboxylic Acid Derivatives and (3-Arylaziridin-2-yl)ketones: The Aziridination Approaches.Int J Mol Sci. 2021 Sep 13;22(18):9861. doi: 10.3390/ijms22189861. Int J Mol Sci. 2021. PMID: 34576025 Free PMC article. Review.
-
Recent Advances in the Catalytic Asymmetric Reactions of Oxaziridines.Molecules. 2018 Oct 16;23(10):2656. doi: 10.3390/molecules23102656. Molecules. 2018. PMID: 30332802 Free PMC article. Review.
Cited by
-
A modular and divergent approach to spirocyclic pyrrolidines.Chem Sci. 2020 Aug 7;11(38):10354-10360. doi: 10.1039/d0sc03676e. Chem Sci. 2020. PMID: 34094297 Free PMC article.
-
Advances in the chemistry of oxaziridines.Chem Rev. 2014 Aug 27;114(16):8016-36. doi: 10.1021/cr400611n. Epub 2014 Apr 22. Chem Rev. 2014. PMID: 24754443 Free PMC article. No abstract available.
-
Diradical-singlet character of 1,3-dipoles affects reactivity of 1,3-dipolar cycloaddition reactions and intramolecular cyclization.J Mol Model. 2019 Sep 7;25(10):306. doi: 10.1007/s00894-019-4162-9. J Mol Model. 2019. PMID: 31494732
-
Dipolar addition to cyclic vinyl sulfones leading to dual conformation tricycles.Beilstein J Org Chem. 2013 Jul 15;9:1419-25. doi: 10.3762/bjoc.9.159. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 23946837 Free PMC article.
-
Iron catalyzed asymmetric oxyamination of olefins.J Am Chem Soc. 2012 Aug 1;134(30):12370-3. doi: 10.1021/ja3046684. Epub 2012 Jul 23. J Am Chem Soc. 2012. PMID: 22793789 Free PMC article.
References
-
-
For reviews of nitrone cycloaddition chemistry, see: Revuelta J, Cicchi S, Goti A, Brandi A. Synthesis. 2007:485–504. Merino P. In: Science of Synthesis. Padwa A, editor. Vol. 27. Stuttgart: Thieme; 2004. pp. 511–580. Gothelf KV, Jørgensen KA. Chem. Commun. 2000:1449–1458. Frederickson M. Tetrahedron. 1997;53:403–425. Confalone PN, Huie EM. Org. React. 1988;36:1–173. Tufariello JJ, J J. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. Vol. 2. Chichester: John Wiley & Sons; 1984. pp. 83–168. Black DS, Crozier RF, Davis VC. Synthesis. 1975:205–221.
-
-
-
For recent reviews of azide cycloaddition chemistry, see: Best MD. Biochemistry. 2009;48:6571–6584. Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952–3015. Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med. Res. Rev. 2008;28:278–308. Gil MV, Arévalo MJ, López Ó. Synthesis. 2007:1589–1620. Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. Lutz J-F. Angew. Chem. Int. Ed. 2007;46:1018–1025. Wu P, Fokin VV. Aldrichimica Acta. 2007;40:7–17. Binder WH, Kluger C. Curr. Org. Chem. 2006;10:1791–1815. Bock VD, Hiemstra H, Maarseveen JH. Eur. J. Org. Chem. 2006:51–68.
-
-
-
For general reviews of 1,3-dipolar cycloadditions, see: Stanley LM, Sibi MP. Chem. Rev. 2008;108:2887–2902. Nair V, Suja TD. Tetrahedron. 2007;63:12247–12275. Pellissier H. Tetrahedron. 2007;63:3235–3285. Molteni G. Heterocycles. 2006;68:2177–2202. Koumbis AE, Gallos JK. Curr. Org. Chem. 2003;7:771–797. Koumbis AE, Gallos JK. Curr. Org. Chem. 2003;7:585–628. Koumbis AE, Gallos JK. Curr. Org. Chem. 2003;7:397–425. Padwa A, Pearson WH, editors. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. New York: Wiley; 2002. Karlsson S, Högberg H-E. Org. Prep. Proced. Int. 2001;33:105–172. Gothelf KV, Jørgensen KA. Chem. Rev. 1998;98:863–909.
-
-
- Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:633–696.
- Huisgen R. Angew. Chem. 1963;75:742–754.
- Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:565–598.
- Huisgen R. Angew. Chem. 1963;75:604–637.
-
-
Azimines: Challand SR, Gait SF, Rance MJ, Rees CW, Storr RC. J. Chem. Soc., Perkin Trans. 1975;1:26–31. Gait SF, Rance MJ, Rees CW, Storr RC. J. Chem. Soc., Chem. Commun. 1972:688–689. Nitroso oxides: c) Ishikawa S, Nojima T, Sawaki Y. J. Chem. Soc., Perkin Trans. 1996;2:127–132. Ishikawa S, Tsuji S, Sawaki Y. J. Am. Chem. Soc. 1991;113:4282–4288.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

