Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors
- PMID: 20029995
- PMCID: PMC2927132
- DOI: 10.1002/cne.22236
Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors
Abstract
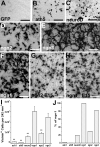
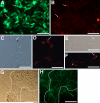
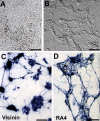
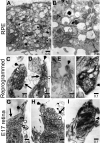
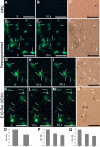
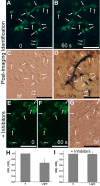
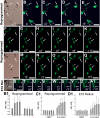
Photoreceptors are highly specialized sensory neurons in the retina, and their degeneration results in blindness. Replacement with developing photoreceptor cells promises to be an effective therapy, but it requires a supply of new photoreceptors, because the neural retina in human eyes lacks regeneration capability. We report efficient generation of differentiating, photoreceptor-like neurons from chick retinal pigment epithelial (RPE) cells propagated in culture through reprogramming with neurogenin1 (ngn1). In reprogrammed culture, a large number of the cells (85.0% +/- 5.9%) began to differentiate toward photoreceptors. Reprogrammed cells expressed transcription factors that set in motion photoreceptor differentiation, including Crx, Nr2E3, NeuroD, and RXRgamma, and phototransduction pathway components, including transducin, cGMP-gated channel, and red opsin of cone photoreceptors (equivalent to rhodopsin of rod photoreceptors). They developed inner segments rich in mitochondria. Furthermore, they responded to light by decreasing their cellular free calcium (Ca(2+)) levels and responded to 9-cis-retinal by increasing their Ca(2+) levels after photobleaching, hallmarks of photoreceptor physiology. The high efficiency and the advanced photoreceptor differentiation indicate ngn1 as a gene of choice to reprogram RPE progeny cells to differentiate into photoreceptor neurons in future cell replacement studies.
2009 Wiley-Liss, Inc.
Figures


Similar articles
-
Using neurogenin to reprogram chick RPE to produce photoreceptor-like neurons.Invest Ophthalmol Vis Sci. 2010 Jan;51(1):516-25. doi: 10.1167/iovs.09-3822. Epub 2009 Jul 23. Invest Ophthalmol Vis Sci. 2010. PMID: 19628733 Free PMC article.
-
Photoreceptor-like cells from reprogramming cultured mammalian RPE cells.Mol Vis. 2013 May 30;19:1178-87. Print 2013. Mol Vis. 2013. PMID: 23734087 Free PMC article.
-
Derivation of human differential photoreceptor-like cells from the iris by defined combinations of CRX, RX and NEUROD.PLoS One. 2012;7(4):e35611. doi: 10.1371/journal.pone.0035611. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558175 Free PMC article.
-
Generating retinal neurons by reprogramming retinal pigment epithelial cells.Expert Opin Biol Ther. 2010 Aug;10(8):1227-39. doi: 10.1517/14712598.2010.495218. Expert Opin Biol Ther. 2010. PMID: 20528097 Free PMC article. Review.
-
Loss and gain of cone types in vertebrate ciliary photoreceptor evolution.Dev Biol. 2017 Nov 1;431(1):26-35. doi: 10.1016/j.ydbio.2017.08.038. Epub 2017 Sep 4. Dev Biol. 2017. PMID: 28882401 Review.
Cited by
-
Choice of Cell Source in Cell-Based Therapies for Retinal Damage due to Age-Related Macular Degeneration: A Review.J Ophthalmol. 2013;2013:465169. doi: 10.1155/2013/465169. Epub 2013 Apr 22. J Ophthalmol. 2013. PMID: 23710332 Free PMC article.
-
Direct conversion of adult human retinal pigmented epithelium cells to neurons with photoreceptor properties.Exp Biol Med (Maywood). 2021 Jan;246(2):240-248. doi: 10.1177/1535370220963755. Epub 2020 Oct 18. Exp Biol Med (Maywood). 2021. PMID: 33070653 Free PMC article.
-
The Retinal Pigment Epithelium: a Convenient Source of New Photoreceptor cells?J Ophthalmic Vis Res. 2014 Jan;9(1):83-93. J Ophthalmic Vis Res. 2014. PMID: 24982737 Free PMC article.
-
Photoreceptor-like cells in transgenic mouse eye.Invest Ophthalmol Vis Sci. 2013 Jul 16;54(7):4766-75. doi: 10.1167/iovs.13-11936. Invest Ophthalmol Vis Sci. 2013. PMID: 23847312 Free PMC article.
-
Chick retinal pigment epithelium transdifferentiation assay for proneural activities.Methods Mol Biol. 2012;884:201-9. doi: 10.1007/978-1-61779-848-1_14. Methods Mol Biol. 2012. PMID: 22688708 Free PMC article.
References
-
- Adler R, Curcio C, Hicks D, Price D, Wong F. Cell death in age-related macular degeneration. Mol Vis. 1999;5:31. - PubMed
-
- Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. - PubMed
-
- Aramant RB, Seiler MJ. Retinal transplantation--advantages of intact fetal sheets. Prog Retin Eye Res. 2002;21:57–73. - PubMed
-
- Austin CP, Feldman DE, Ida JA, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

