Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity
- PMID: 20030365
- PMCID: PMC2834186
- DOI: 10.1021/np900622m
Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity
Abstract
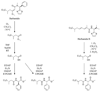
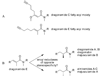
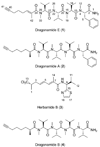
Tropical parasitic and infectious diseases, such as leishmaniasis, pose enormous global health threats, but are largely neglected in commercial drug discovery programs. However, the Panama International Cooperative Biodiversity Group (ICBG) has been working to identify novel treatments for malaria, Chagas' disease, and leishmaniasis through an investigation of plants and microorganisms from Panama. We have pursued activity-guided isolation from an extract of Lyngbya majuscula that was found to be active against leishmaniasis. A new modified linear peptide from the dragonamide series was isolated, dragonamide E (1), along with two known modified linear peptides, dragonamide A (2) and herbamide B (3). Dragonamides A and E and herbamide B exhibited antileishmanial activity with IC50 values of 6.5, 5.1, and 5.9 microM, respectively. Spectroscopic and stereochemical data for dragonamide E (1) and herbamide B (3; the spectroscopic and stereochemical data for this substance is incomplete in the literature) are presented as well as comparisons of biological activity within the dragonamide compound family. Biosynthetic differences among marine compounds with a terminal free amide are also discussed.
Figures

Similar articles
-
Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula.J Nat Prod. 2007 Jun;70(6):984-8. doi: 10.1021/np0700772. Epub 2007 Apr 19. J Nat Prod. 2007. PMID: 17441769 Free PMC article.
-
Malyngolide dimer, a bioactive symmetric cyclodepside from the panamanian marine cyanobacterium Lyngbya majuscula.J Nat Prod. 2010 Apr 23;73(4):709-11. doi: 10.1021/np9005184. J Nat Prod. 2010. PMID: 20158242 Free PMC article.
-
Dragonamides C and D, linear lipopeptides from the marine cyanobacterium brown Lyngbya polychroa.J Nat Prod. 2008 May;71(5):887-90. doi: 10.1021/np0706769. Epub 2008 Apr 5. J Nat Prod. 2008. PMID: 18393465
-
Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: isolation, stereostructure, and biological activity.Org Lett. 2009 Nov 5;11(21):5062-5. doi: 10.1021/ol9020546. Org Lett. 2009. PMID: 19803465
-
Exploring microalgal and cyanobacterial metabolites with antiprotozoal activity against Leishmania and Trypanosoma parasites.Acta Trop. 2024 Mar;251:107116. doi: 10.1016/j.actatropica.2023.107116. Epub 2023 Dec 28. Acta Trop. 2024. PMID: 38159713 Review.
Cited by
-
New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade.Mar Drugs. 2017 May 5;15(5):132. doi: 10.3390/md15050132. Mar Drugs. 2017. PMID: 28475149 Free PMC article. Review.
-
Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects.Front Microbiol. 2017 Apr 25;8:515. doi: 10.3389/fmicb.2017.00515. eCollection 2017. Front Microbiol. 2017. PMID: 28487674 Free PMC article. Review.
-
Marine Cyanobacteria: A Rich Source of Structurally Unique Anti-Infectives for Drug Development.Molecules. 2024 Nov 10;29(22):5307. doi: 10.3390/molecules29225307. Molecules. 2024. PMID: 39598696 Free PMC article. Review.
-
Protective efficacy of Chlorophytum borivilianum root extract against murine visceral leishmaniasis by immunomodulating the host responses.J Ayurveda Integr Med. 2020 Jan-Mar;11(1):53-61. doi: 10.1016/j.jaim.2017.10.009. Epub 2018 Aug 14. J Ayurveda Integr Med. 2020. PMID: 30120057 Free PMC article.
-
Cyanobacteria, Lyngbya aestuarii and Aphanothece bullosa as antifungal and antileishmanial drug resources.Asian Pac J Trop Biomed. 2013 Jun;3(6):458-63. doi: 10.1016/S2221-1691(13)60096-9. Asian Pac J Trop Biomed. 2013. PMID: 23730558 Free PMC article.
References
-
- Pink R, Hudson A, Mouries MA, Bendig M. Nat. Rev. Drug Discov. 2005;4:727–740. - PubMed
-
- Nwaka S, Hudson A. Nat. Rev. Drug Discov. 2006;5:941–955. - PubMed
-
- Capson TL, Coley PD, Kursar TA. Nat. Biotechnol. 1996;14:1200–1202. - PubMed
-
- Kursar TA, Capson TL, Coley PD, Corley DG, Gupta MB, Harrison LA, Ortega-Barría E, Windsor DM. Pharm. Biol. 1999;37(Suppl):114–126.
-
- Coley PD, Heller MV, Aizprua R, Araúz B, Flores N, Correa M, Gupta M, Solis PN, Ortega-Barría E, Romero LI, Gómez B, Ramos M, Cubilla-Rios L, Capson TL, Kursar TA. Front. Ecol. Environ. 2003;1:421–428.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

