Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability
- PMID: 20030375
- PMCID: PMC2846717
- DOI: 10.1021/bi901810u
Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability
Abstract
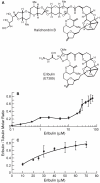
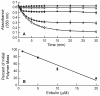
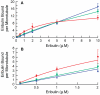
Eribulin mesylate (E7389), a synthetic analogue of the marine natural product halichondrin B, is in phase III clinical trials for the treatment of cancer. Eribulin targets microtubules, suppressing dynamic instability at microtubule plus ends through an inhibition of microtubule growth with little or no effect on shortening [Jordan, M. A., et al. (2005) Mol. Cancer Ther. 4, 1086-1095]. Using [(3)H]eribulin, we found that eribulin binds soluble tubulin at a single site; however, this binding is complex with an overall K(d) of 46 microM, but also showing a real or apparent very high affinity (K(d) = 0.4 microM) for a subset of 25% of the tubulin. Eribulin also binds microtubules with a maximum stoichiometry of 14.7 +/- 1.3 molecules per microtubule (K(d) = 3.5 microM), strongly suggesting the presence of a relatively high-affinity binding site at microtubule ends. At 100 nM, the concentration that inhibits microtubule plus end growth by 50%, we found that one molecule of eribulin is bound per two microtubules, indicating that the binding of a single eribulin molecule at a microtubule end can potently inhibit its growth. Eribulin does not suppress dynamic instability at microtubule minus ends. Preincubation of microtubules with 2 or 4 microM vinblastine induced additional lower-affinity eribulin binding sites, most likely at splayed microtubule ends. Overall, our results indicate that eribulin binds with high affinity to microtubule plus ends and thereby suppresses dynamic instability.
Figures


References
-
- Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsk BM, Palme MH, Habgood GJ, Singer LA, Dipietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. - PubMed
-
- Dabydeen DA, Burnett JC, Bai RL, Verdier-Pinard P, Hickford SJH, Pettit GR, Blunt JW, Munro MHG, Gussio R, Hamel E. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Molecular Pharmacology. 2006;70:1866–1875. - PubMed
-
- Hirata Y, Uemura D. Halichondrins - Antitumor Polyether Macrolides from a Marine Sponge. Pure and Applied Chemistry. 1986;58:701–710.
-
- Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, Mani S, Takimoto CH. A Phase I Study of Eribulin Mesylate (E7389), a Mechanistically Novel Inhibitor of Microtubule Dynamics, in Patients with Advanced Solid Malignancies. Clinical Cancer Research. 2009;15:4207–4212. - PubMed
-
- Tan AR, Rubin EH, Walton DC, Shuster DE, Wong YN, Fang F, Ashworth S, Rosen LS. Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4213–4219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

