Decoding the nitrogenase mechanism: the homologue approach
- PMID: 20030377
- PMCID: PMC2840065
- DOI: 10.1021/ar900254x
Decoding the nitrogenase mechanism: the homologue approach
Abstract
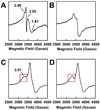
The (Mo)-nitrogenase is a complex metalloenzyme that catalyzes the key step in the global nitrogen cycle, the reduction of atmospheric dinitrogen (N(2)) to bioavailable ammonia (NH(3)), at the iron-molybdenum cofactor (FeMoco) site of its molybdenum-iron (MoFe) protein component. Despite the fundamental significance of biological nitrogen fixation and extensive studies over the past decades, the catalytic mechanism of nitrogenase has not been deciphered. One major challenge for the mechanistic study of nitrogenase is the redox versatility of its FeMoco center. The ability of FeMoco to shuttle between oxidation states in a rapid and unsynchronized manner results in a mixed oxidation state of the cofactor population during turnover. The substrate and the various intermediates can only interact with the FeMoco site in a transient manner, so it is extremely difficult to capture any substrate- or intermediate-bound form of nitrogenase for the direct examination of substrate-enzyme interactions during catalysis. In this Account, we describe the approach of identifying a partially "defective" nitrogenase homologue, one with a slower turnover rate, as a means of overcoming this problem. The NifEN protein complex serves as an ideal candidate for this purpose. It is an alpha(2)beta(2)-heterotetramer that contains cluster-binding sites homologous to those found in the MoFe protein: the "P-cluster site" at the interface of the alphabeta-subunit dimer, which accommodates a [Fe(4)S(4)]-type cluster; and the "FeMoco site" within the alpha-subunit, which houses an all-iron homologue to the FeMoco. Moreover, NifEN mimics the MoFe protein in catalysis: it is capable of reducing acetylene (C(2)H(2)) and azide (N(3)(-)) in an ATP- and iron (Fe) protein-dependent manner. However, NifEN is unable to reduce proton (H(+)) and N(2), and it is an inefficient enzyme with a restricted electron flux during the turnover. The extremely slow turnover rate of NifEN and the possible "synchronization" of its FeMoco homologue at a certain oxidation level permit the observation of a new S = 1/2 EPR signal upon turnover of C(2)H(2) by NifEN, which is analogous to the signal reported for a MoFe protein variant upon turnover of the same substrate. This result is exciting, because it suggests the possibility of naturally enriching a C(2)H(2)-bound form of NifEN for the successful crystallization of the first intermediate-bound nitrogenase homologue. On the other hand, the fact that NifEN represents a partially "defective" homologue of the MoFe protein makes it a promising mutational platform on which a functional MoFe protein equivalent may be reconstructed by introducing the missing features of MoFe protein step-by-step into NifEN. Such a strategy allows us to define the function of each feature and address questions such as the following: What is the function of P-cluster in catalysis? Are Mo and homocitrate the essential constituents of the cofactor in N(2) reduction? How does substrate accessibility affect the reactivity of the enzyme? This homologue approach could complement the mechanistic analysis of the nitrogenase MoFe protein, and information derived from both approaches will help achieve the ultimate goal of solving the riddle of biological nitrogen fixation.
Figures



Similar articles
-
NifEN: a versatile player in nitrogenase assembly, catalysis and evolution.J Biol Inorg Chem. 2025 Mar;30(2):135-149. doi: 10.1007/s00775-024-02086-6. Epub 2024 Dec 12. J Biol Inorg Chem. 2025. PMID: 39663240 Review.
-
Formation of a homocitrate-free iron-molybdenum cluster on NifEN: implications for the role of homocitrate in nitrogenase assembly.Dalton Trans. 2010 Mar 28;39(12):3124-30. doi: 10.1039/c000264j. Epub 2010 Feb 18. Dalton Trans. 2010. PMID: 20221547 Free PMC article.
-
Catalytic activities of NifEN: implications for nitrogenase evolution and mechanism.Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):16962-6. doi: 10.1073/pnas.0907872106. Epub 2009 Sep 23. Proc Natl Acad Sci U S A. 2009. PMID: 19805110 Free PMC article.
-
Dual functions of NifEN: insights into the evolution and mechanism of nitrogenase.Dalton Trans. 2010 Mar 28;39(12):2964-71. doi: 10.1039/b922555b. Epub 2010 Jan 11. Dalton Trans. 2010. PMID: 20221527 Free PMC article.
-
Mechanism of Mo-dependent nitrogenase.Annu Rev Biochem. 2009;78:701-22. doi: 10.1146/annurev.biochem.78.070907.103812. Annu Rev Biochem. 2009. PMID: 19489731 Free PMC article. Review.
Cited by
-
X-ray Magnetic Circular Dichroism Spectroscopy Applied to Nitrogenase and Related Models: Experimental Evidence for a Spin-Coupled Molybdenum(III) Center.Angew Chem Int Ed Engl. 2019 Jul 8;58(28):9373-9377. doi: 10.1002/anie.201901899. Epub 2019 Jun 18. Angew Chem Int Ed Engl. 2019. PMID: 31119827 Free PMC article.
-
Mechanism of nitrogen fixation by nitrogenase: the next stage.Chem Rev. 2014 Apr 23;114(8):4041-62. doi: 10.1021/cr400641x. Epub 2014 Jan 27. Chem Rev. 2014. PMID: 24467365 Free PMC article. Review. No abstract available.
-
The Spectroscopy of Nitrogenases.Chem Rev. 2020 Jun 24;120(12):5005-5081. doi: 10.1021/acs.chemrev.9b00650. Epub 2020 Apr 2. Chem Rev. 2020. PMID: 32237739 Free PMC article. Review.
-
NifEN: a versatile player in nitrogenase assembly, catalysis and evolution.J Biol Inorg Chem. 2025 Mar;30(2):135-149. doi: 10.1007/s00775-024-02086-6. Epub 2024 Dec 12. J Biol Inorg Chem. 2025. PMID: 39663240 Review.
-
Iron L2,3-Edge X-ray Absorption and X-ray Magnetic Circular Dichroism Studies of Molecular Iron Complexes with Relevance to the FeMoco and FeVco Active Sites of Nitrogenase.Inorg Chem. 2017 Jul 17;56(14):8147-8158. doi: 10.1021/acs.inorgchem.7b00852. Epub 2017 Jun 27. Inorg Chem. 2017. PMID: 28653855 Free PMC article.
References
-
- Eady RR. Current status of structure function relationships of vanadium nitrogenase. Coordin. Chem. Rev. 2003;237:23–30.
-
- Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chem. Rev. 1996;96:2983–3012. - PubMed
-
- Howard JB, Rees DC. Structural basis of biological nitrogen fixation. Chem. Rev. 1996;96:2965–2982. - PubMed
-
- Barney BM, Lee HI, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC. Breaking the N2 triple bond: insights into the nitrogenase mechanism. Dalton Trans. 2006;19:2277–2284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

